The handling of metal-loaded and exhausted adsorbent from the adsorption process is very important and gives useful information to be allowed for the economic design of overall operation. The desorption can be carried out by means of various mechanism such as adsorption, ion exchange, and complexation, where metals are desorbed from the adsorbent by a proper eluant to produce a small, concentrated volume of metal-containing solution, from which metals could potentially be recovered [1]. Studies have shown that the protons of strong acids such as HCl, H2SO4 and HNO3 can be replaced with metals bound to the sorbents [2,3]. Desorption can be also carried out by chelating agents (EDTA) or exchange with other ions (i.e. CaCl2)[4,5]. The decision of desorbing agent depends on the property or characteristic of adsorbent used and the metals adsorbed. The best desorbing agent is one that elutes completely target metal without deteriorating the property of the adsorbent. After elution, concentrated metals can be recovered by electrochemical or other conventional techniques [6].
The regeneration process can be necessary to maximize function and capability of adsorbent used. Also, ability of regeneration for expensive and selective sorbent is especially very important in both batch and continuous processes.
We have already shown that nickel ions could be entirely adsorbed by sericite and the adsorbed nickel ions could be simply and completely eluted by HNO3[7]. Until now, there is no study on desorption for nickel ions adsorbed onto sericite. Therefore, the aim of this work was to investigate desorption and regeneration characteristics for nickel ions previously adsorbed onto sericite under various experimental conditions.
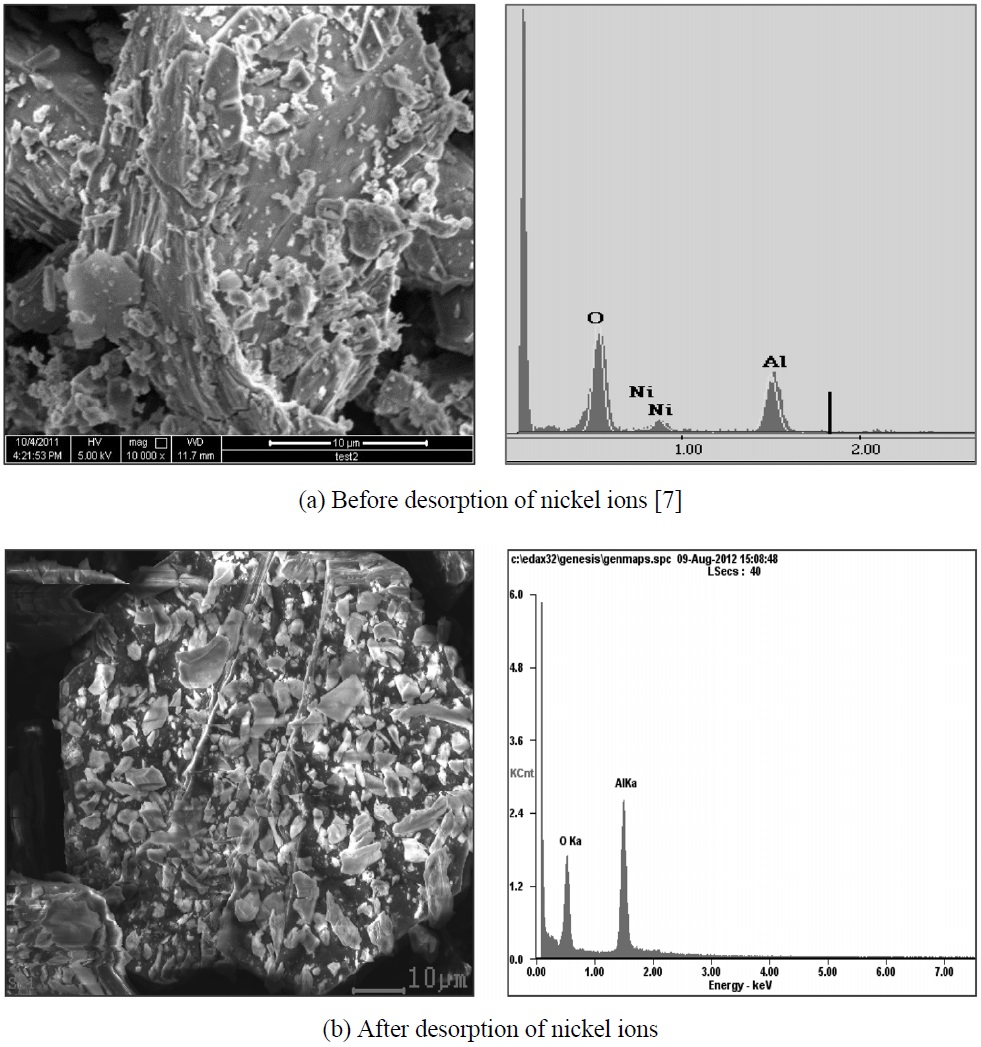
We have already chosen HNO3 as best desorbing agent in the previous work [7]. The SEM (Scanning electron microscopy, Hitachi model S-4100, Japan) photograph and EDX (Energy dispersive X-ray spectroscopy, U.S.A) were used to confirm whether desorption for sericite of nickel ions was occurred or not.
For the further desorption experiment to investigate effect of various experimental conditions such as concentration, S/L ratio which is defined as the ratio of adding amount of adsorbent and volume of desorbing agent, and contact time on desorption efficiency was performed. Previously adsorbed nickel ions on sericite was added to a flask including 100 mL of HNO3 .The mixture was shaken at 250 rpm using a rotary shaking incubator (JEIO TECH, SI-600R, Korea) at room temperature. The desorption efficiency of nickel ions from the sericite was calculated as the ratio between the amount of nickel ions desorbed and amount of nickel ions adsorbed. In case of regeneration experiment, sericite which metal was eluted to the HNO3 solution was thoroughly washed three times with deionized water to remove any traces of HNO3 solution, and then mixed again in waste water containing nickel ions for the next adsorption cycle. The above procedure was employed for four consecutive cycles. The removal efficiency of nickel ions for each cycle was calculated as the following equation:
R.E = (Ci - Cf) / Ci
where R.E is the removal efficiency of nickel ions (%), Ci is the initial concentration of nickel ions (mg/L), and Cf is the final concentration of nickel ions (mg/L). The concentration for nickel ions was analyzed by Atomic Absorption Spectroscopy (Perkin-Elmer A Analyst 100/A Analyst 700, U.S.A). All experiment was performed three times and the average value was presented.
To confirm surface condition for before and after desorption of nickel ions, SEM photograph and EDX spectrum was applied. As shown in Figure 1, the peak of nickel ions was not appeared at the EDX spectrum (b) as compared with (a). From the result, it was proved that nickel ion was clearly eluted from sericite using HNO3 solution.
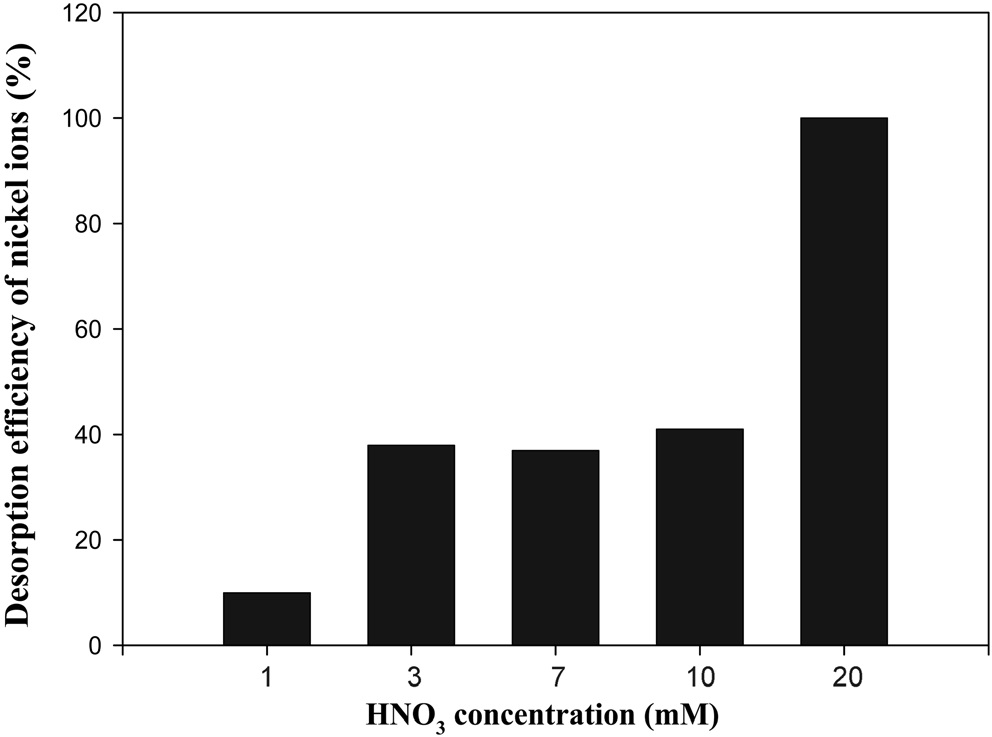
To investigate the best experimental condition for desorption process using HNO3, effect of HNO3 concentration, S/L ratio, and desorption time on desorption efficiency for nickel ions were considered. Firstly, the experiment to find the optimal concentration
of HNO3 was done. Various concentrations of HNO3 with 1, 3, 7, 10 and 20 mM were tried to elute nickel ions adsorbed onto sericite. Loading amount for nickel ions of sericite was 44 mg/g dry mass [7]. Also, desorption time was fixed as 60min. As shown in Figure 2, desorption efficiency for nickel ions increased as the concentration of HNO3 increased. Especially, all nickel ions was entirely eluted at the 20 mM of concentration. Therefore, further experiment was not done in the range over 20 mM of concentration and the concentration was chosen as the optimal concentration for HNO3 in terms of efficiency.
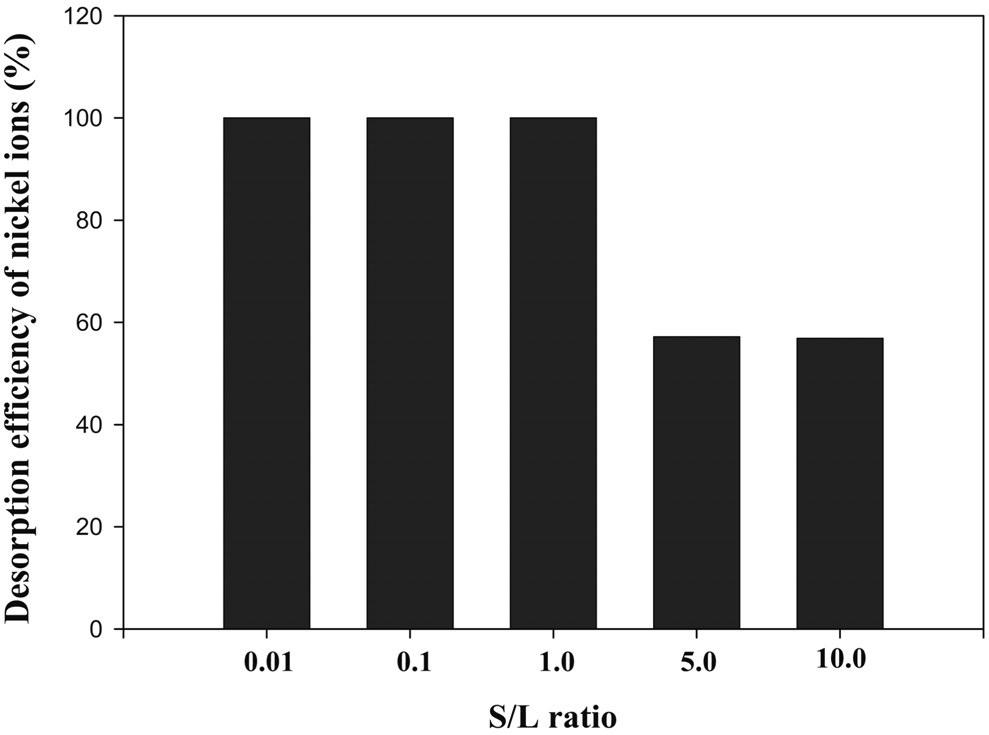
Meanwhile, the S/L ratio is also very important operation parameter in batch processes, especially [5]. Various S/L ratios including 0.01, 0.1, 1.0, 5.0 and 10.0 were tested, respectively. Figure 3 shows that desorption efficiency decreased as S/L ratio
increased. Especially, desorption efficiency was 100% in the range of 0.01-1.0. It can be explained that a lot of desorbing agent was used for comparatively less sericite. Generally, it is well known that desorption efficiency increased as the S/L ratio decreased [9]. However, desorption efficiency for nickel ions was very low by about 54% in the range over 5.0 of S/L ratio. In terms of the cost effect, there is the best value for S/L ratio. Generally, to apply desorption operation to real processes, concentration factor (data not shown) which is defined as the ratio of desorbed metal concentration in desorbing agent solution and the initial metal concentration in aqueous solution must be also considered as another important parameter, at the same time[10].
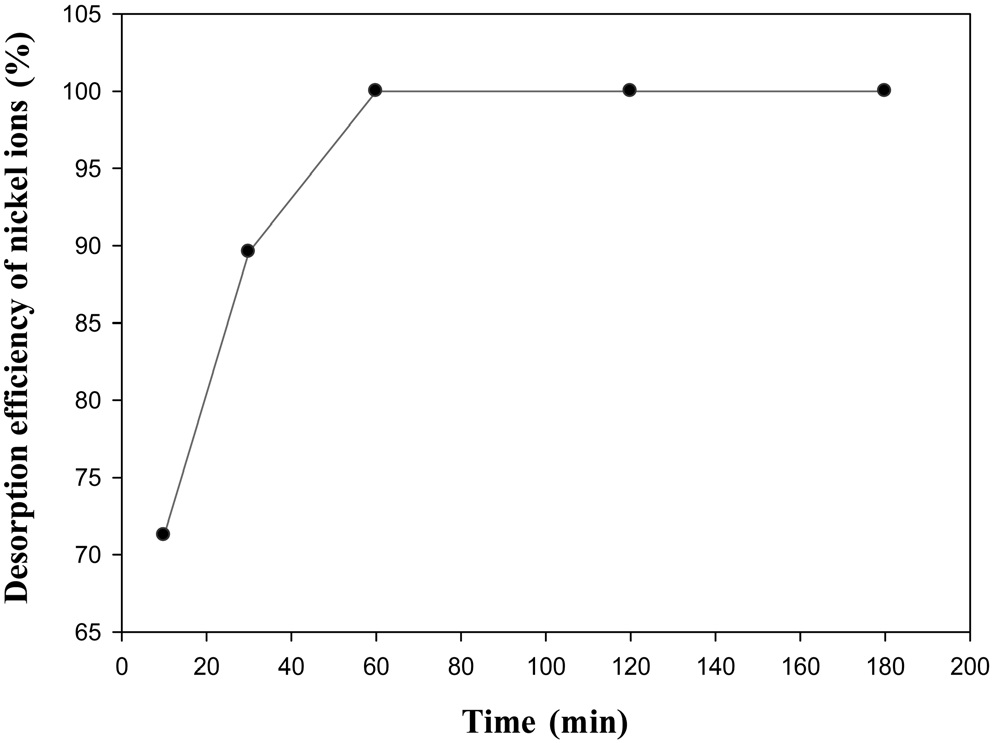
Figure 4 shows that desorption efficiency for nickel ions increased as time increased. However, most of desorption process was completed within 60 min. It means that desorption process was done very quickly. Generally, it was reported that most of the desorption processes were finished within 180 min [11].
To develop a batch treatment process, it is essential to describe regeneration aspects of the process in order to improve its cost-effectiveness by recycling the adsorbent for reuse in multiple
Regeneration of sericite for nickel ions (Initial concentration of nickel ions: 100 mg/L, Sericite concentration: 1 g/L, HNO3 concentration: 20 mM, Final pH: 7.5)
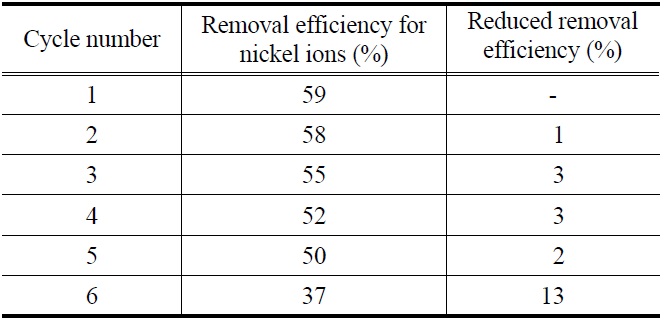
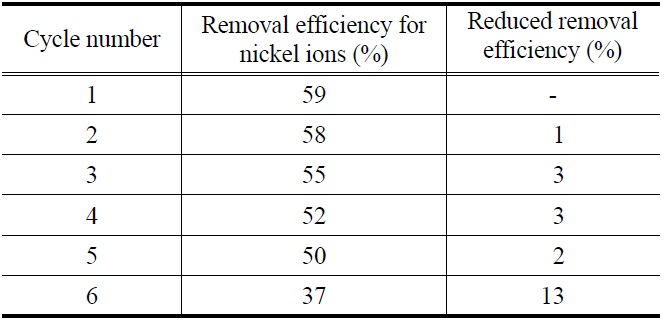
cycles [12]. To investigate regeneration ability for sericite used, sequential adsorption-desorption cycles were repeated 6 times by the means of same adsorbent. As shown in Table 1, removal efficiency of recycled sericite for nickel ions was constantly maintained at the 5th cycle although there is a slight decreasing. It shows that sericite can be sufficiently reused in real process such as industrial wastewater including nickel ions.
To investigate the optimal elution condition for nickel ions adsorbed on sericite, various experimental parameters such as concentration, S/L ratio, and contact time with HNO3 solution was considered. All nickel ions was completely eluted at the 20 mM of HNO3 concentration. And, desorption efficiency for nickel ions was 100% in the range of 0.01~1.0 of S/L ratio. Also, all nickel ions was entirely eluted within 60 min. Elution of nickel ions adsorbed onto sericite using HNO3 solution was confirmed by means of SEM & EDX analysis. Finally, removal efficiency of recycled sericite for nickel ions was constantly maintained at the 4th cycle although there is a slight decreasing.