광학 및 형광 현미경을 이용한 실시간 촉매 모니터링 기술을 활용하여 C5 파라핀과 올레핀의 방향족화 반응 중 H-ZSM-5 촉매 표면에서 발생하는 코크 형성 과정을 시간별, 촉매 위치별로 관찰하였다. 실시간 자외-가시광 분광현미경(
In hydrocarbon processing, formation of carbonaceous materials on working catalysts is usually inevitable and the undesired coke deposition generally provokes loss of activity and change in selectivity. Therefore, a great number of studies have been carried out to understand the nature of coke and thus to find efficient methods preventing or alleviating the unfavorable catalyst deactivation[1,2]. However, the nature of the carbonaceous species is still a matter of debate and the subject of many investigations. Although various characterization techniques,
Aromatization of paraffin or olefin is one of the important methods to convert excess low-value light hydrocarbons into aromatics which can be used as chemical feedstocks as well as highoctane gasoline blending components[6-8]. However, the severe catalyst deactivation of the attractive reaction should be improved to meet industrial requirement. To achieve the elusive goal, it is clear that more detailed information for the coke formation on working catalyst is necessary. However, the complex reaction mechanism as well as fast coke deposition has been an obstacle for in-depth characterization.
In this context, it is aimed here to present the application of
2.1. Materials and experiments
The large H-ZSM-5 crystallites were provided by ExxonMobil (Machelen, Belgium). The detailed information can be found in previous publications[9-11]. The H-ZSM-5 crystal (Si/Al = 7) has typical dimensions of 100 μm × 20 μm × 20 μm. Representative C5 chemicals such as 2-methyl-butane and 2-methyl-2- butene are supplied from Aldrich and used without further purification. The experiments were performed in an appropriate
2.2. Optical microspectroscopy setup
An Olympus BX41 upright microscope equipped with a 50 × 0.5 NA-high working distance objective was used. A 75 W tungsten lamp provided the illumination. The
2.3.Confocal fluorescence microspectroscopy setup
3.1. In-situ optical microspectroscopy
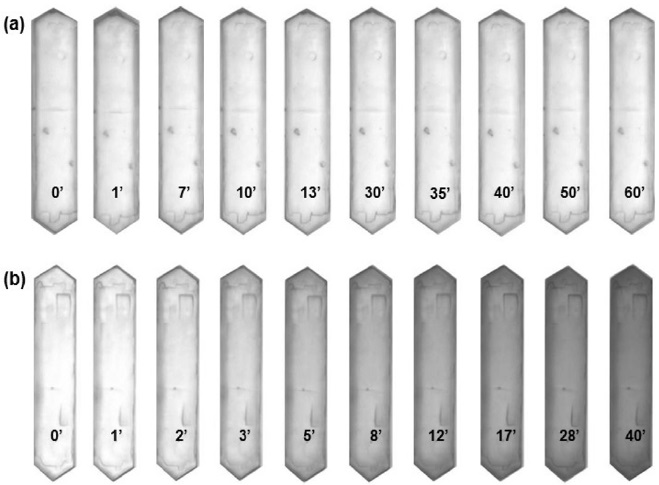
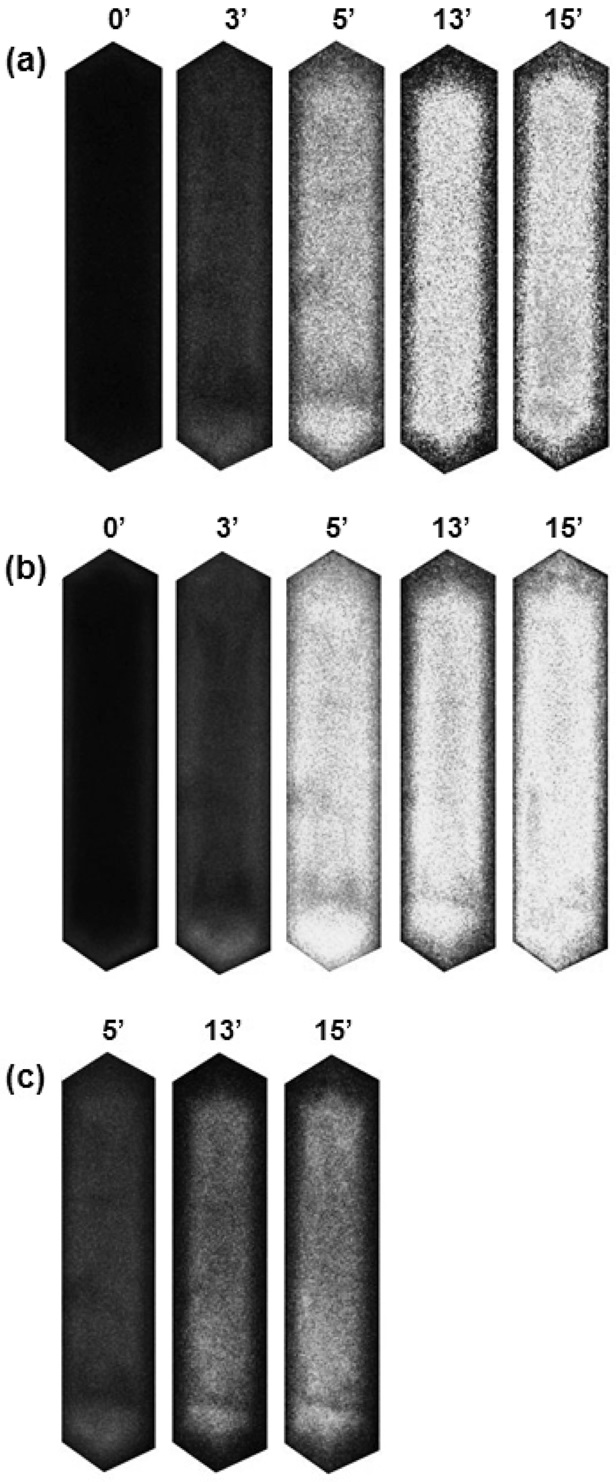
The optical microphotographs presented in Figure 1 show the color change of H-ZSM-5 crystals during the aromatization of representative C5 paraffin (2-methyl-butene) and olefin (2-methyl-2-butene) as a function of time at 773 K. Upon exposure to reactant vapor, the translucent H-ZSM-5 crystals gradually become yellow-brown as the formation of carbonaceous deposition increases.
It is observed that not only the color change rate but also the coloration intensity varies depending on the kind of reactants. With paraffin, the color change rate is slow and the coloration intensity is weak. In the case of olefin, on the other hand, the coloration rate is so fast that the color of H-ZSM-5 crystals turns dark brown or black indicating faster coke deposition.
Time-resolved
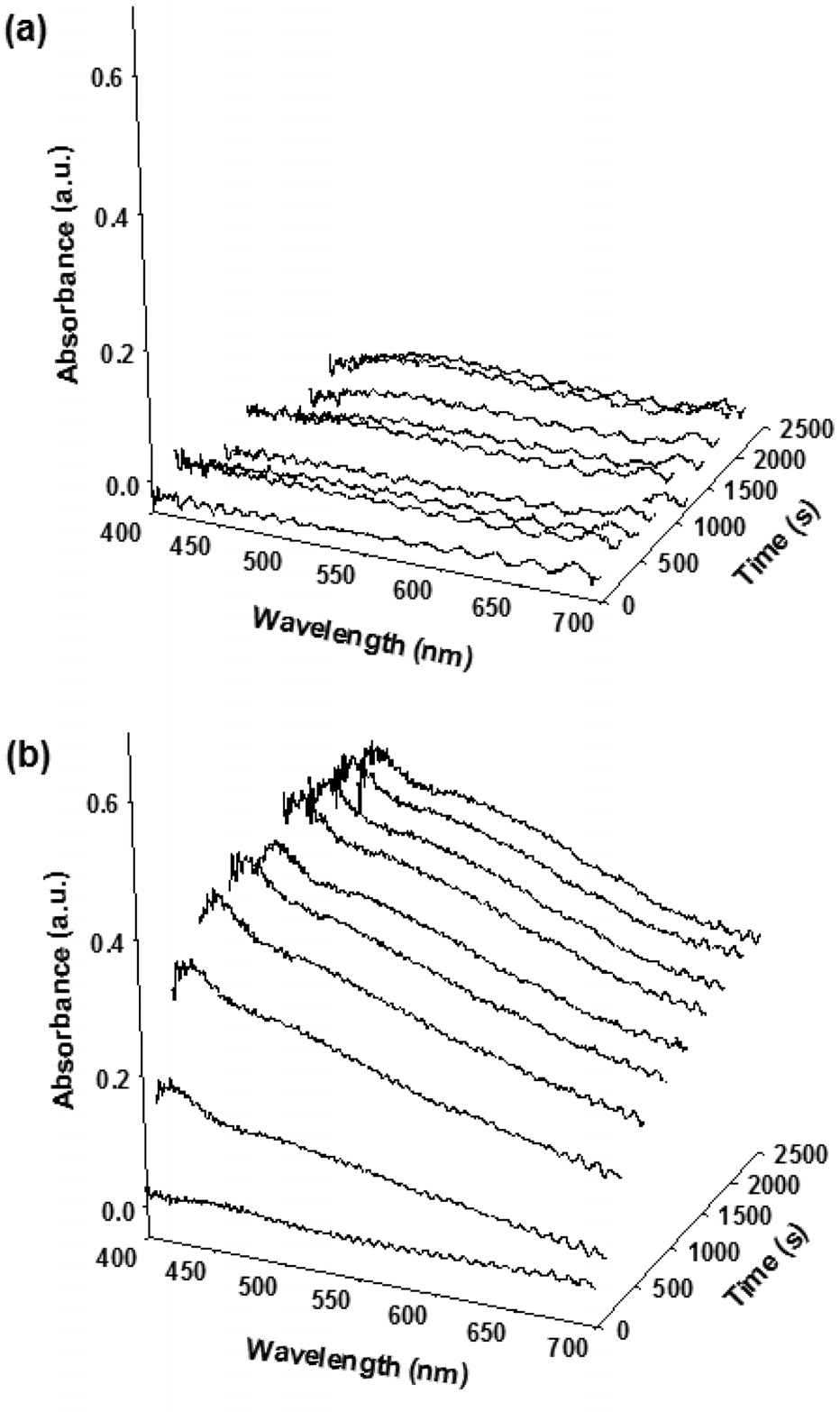
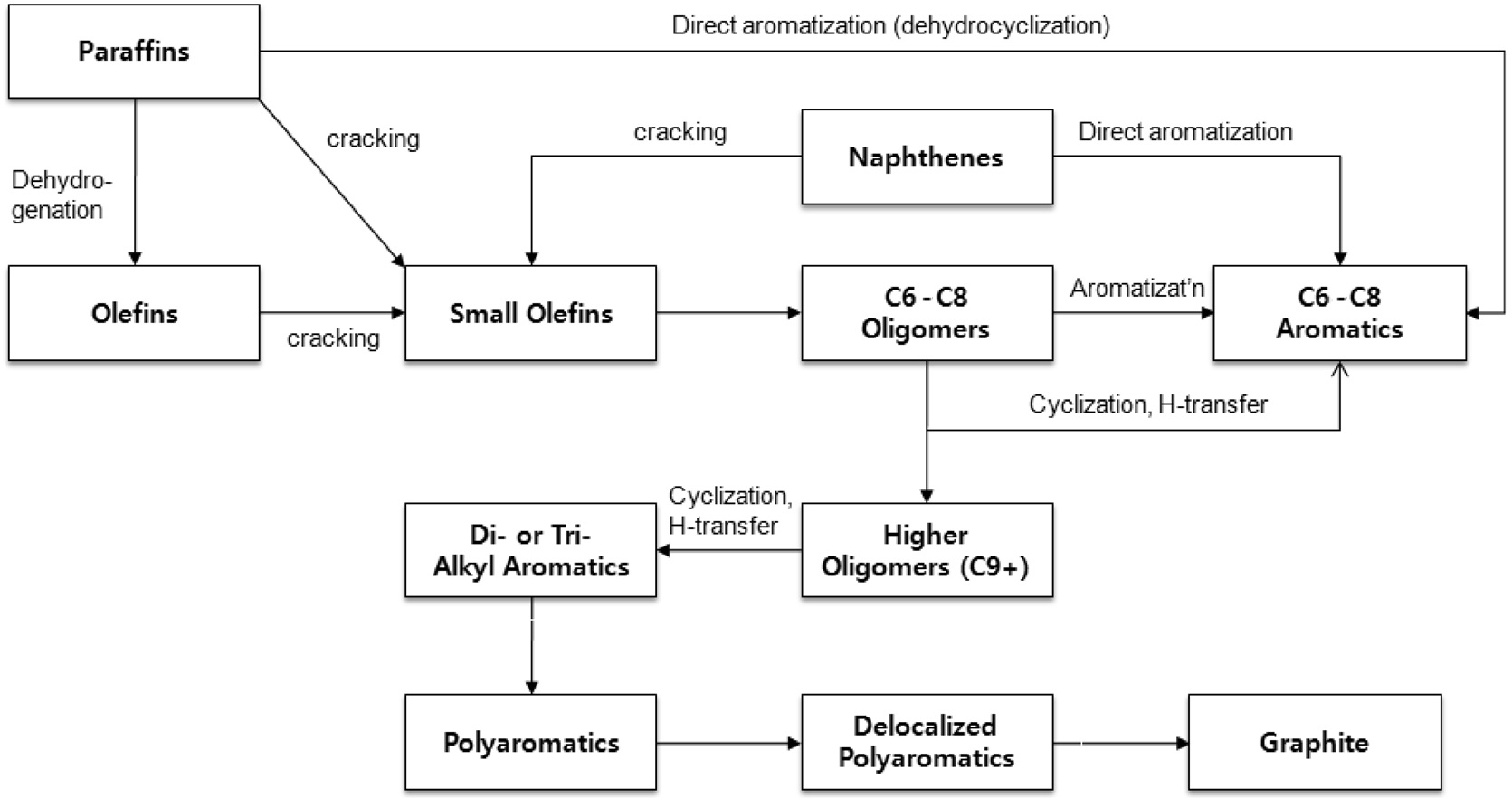
The aromatization reaction begins with the chain growth of small olefins and then the resulting aliphatic olefins transform to light alkyl aromatics by cyclization as described in Figure 3. Considering the reaction pathways of aromatization and coke formation[3,12,13], the slow coke deposition confirmed by the low absorption intensity indicates slow dehydration or cracking of paraffin to smaller olefin within H-ZSM-5 crystals.
Although one can assume that the transformation of paraffin to olefin is fast and olefinic or polyolefinic coke precursor (coke type I) may be formed in the pore, it is not plausible because these polyenylic cations are easily transformed to coke type II (typically small aromatic carbocations and bulky polyaromatics) at high temperature[3]. In addition, it is difficult to confirm the existence of the light cokes due to a limitation in the detection range of optical microscopy (400~700 nm). The optical absorption of the light coke deposition is usually found below 390 nm.
On the other hand, two broad bands around 410 and 550 nm are observed during an aromatization of olefin. The absorption band around 410 nm is assigned to the π - π* transition of lowcondensed aromatics,
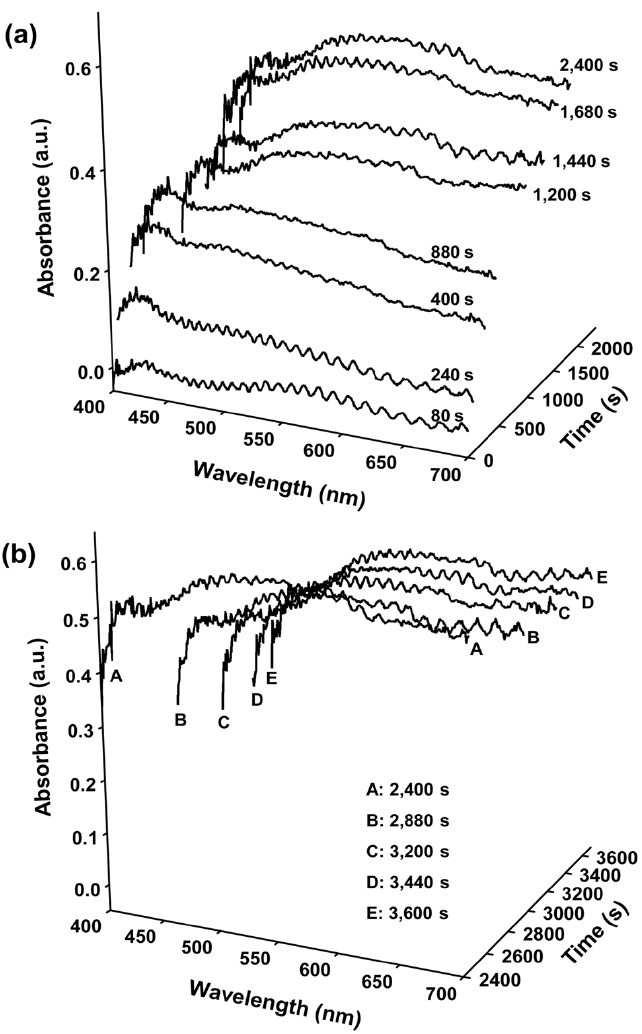
Figure 4 shows the optical absorption spectra of H-ZSM-5 crystals taken from a spot in the crystal edge during an aromatization of olefin. The dominant absorption around 500~580 nm wavelengths as well as stronger background absorption across the whole visible region clearly shows that the coke deposition is more severe in the edge region comprised of straight pores compared to the central region with zigzag pores[9]. The fast coke deposition results in fast catalyst deactivation. In particular, it is noteworthy that the intensity of absorption band around 410 nm changes after ceasing the olefin supply. With continuing olefin supply, the intensity of the band around 410 nm is maintained regardless of the gradual growth of the band around 550 nm. This indicates that the coke growth cycle is maintained by continuous feed supply. In other words, the coke precursors having strong absorption around 410 nm is continuously generated and some part of the species may be transformed to bigger ones identified by the absorption band around 550 nm. After ceasing the olefin supply, it is observed that the band around 550 nm grows up at the expense of the decrease in the absorption around 410 nm (Figure 4(b)).
The red shift supports the transformation of low-condensed aromatics to heavy-condensed ones inside the zeolite crystal. The
3.2. In-situ confocal fluorescence microspectroscopy
stream. The concentration of the fluorescent species excited by 488 nm laser becomes high at the central region, whereas the compound emitting fluorescence by 561 nm laser moves towards the boundary region of the crystal. The different fluorescence phenomena indicate that they correspond to distinct species, most likely, differing in their molecular dimensions. In the present case, it can be due to the different degree of coke growth. The identification of these different coke precursors is difficult at this stage. However, it can be assumed that the former species excited at 488 nm may be lighter coke precursors compared to those excited at 561 nm considering that the fluorescent species photo-excited at 488 nm readily penetrate towards the inner core of the crystal compared to the compounds excited at 561 nm[9]. The red shift in the excitation wavelength indicates a large molecular size.
In line with the previous study[9], the fluorescence profiles during the aromatization can be explained. At the early stage of reaction, light coke precursors are produced and widely distributed within H-ZSM-5 crystal. As time goes on, a portion of the light coke precursors are gradually transformed to more condensed ones. The bigger species either migrate from the internal channels to the channel mouths at the external surfaces or reorganize to fit the channel dimensions because the restricted space in ZSM-5 channels inhibits the formation of bulky polyaromatic compounds[14,16].
The explanation is supported by the fluorescence photograph image which clearly shows that the concentration of lighter coke precursors is high at the central region, whereas larger amount of the bigger carbonaceous species is distributed at the edge region (Figure 5(c)). Once the external surfaces are researched, the carbonaceous species can grow without any steric limitation, giving rise to insoluble “coke” which is responsible for catalyst deactivation by causing pore mouth blocking. These results clearly demonstrate that the
Coke formation during aromatization of paraffin and olefin over H-ZSM-5 zeolite crystal has been investigated in a spaceand time-resolved manner. With optical microphotograph image analysis, it is found that coloration intensities and color change rates of crystals varied depending on the reactant types. In the case of olefin aromatization, faster color change of crystal suggests faster coke deposition compared to that of paraffin.