An increase in environmental awareness and the number of environmental regulations has augmented interest in energy-saving and environmentally friendly materials(Na & Kim, 2012). Composites reinforced with glass or carbon fibers, widely used in the past, have the problem of disposal after their lifetime. In modern polymer technology, a high demand exists for eco-friendly materials. Due to their biodegradable and energy saving properties, biocomposites, made of cellulosic fibers and biodegradable polymers, have recently been applied in extensive studies(Mohanty et al., 2000). Cellulosic fibers have many advantages, including low cost, low density, high strength, and biodegradability. Of these cellulosic fibers, the ramie fiber has good elasticity and specific strength and is commercially available(Hong & Ryu, 1997). Despite these advantages, biocomposites reinforced with cellulosic fibers are not yet widely used as a result of their handicaps, including poor bonding between the cellulosic fiber and the polymer matrix due to the presence of hydroxyl and carboxylic groups and strength reduction of the reinforcement due to the low moisture resistance of cellulosic fibers (Aranberri-Askargorata et al., 2003). Adhesion between the reinforcing fibers and the polymer matrix plays an important role because external stimulation transfer between the matrix and fibers determines the final mechanical properties and reinforcement efficiency. To overcome the drawbacks of cellulose fibers, plasma treatment(Lee et al., 2003; Yuan et al., 2004), ultra-violet irradiation(Halina & Dagmara, 2006; Khan et al., 2006) and electron beam(EB) irradiation have been successfully employed to produce significant physical and chemical changes, as well as changes in the surface structures and surface energies of the fibers. These methods are simple and lead to the formation of functional groups in the hydrophobic polymer chains, resulting in the molecular cross-linking of the cellulose fiber and polymer(Halina et al., 2005; Iller et al., 2002).
The EB irradiation technique is being increasingly utilized to modify the surfaces of various polymer materials, such as fibers, wires, and films. Han et al.(2006) reported EB irradiation is effective in achieving both impurity removal and functional group development on the surface of natural fibers for better bonding with the polymer matrix(Han et al., 2006). Han et al.(2007) also studied cellulose degradation caused by EB irradiation by investigating its viscosity(Han et al., 2007). Dehydrogenation and destruction of an hydroglucose was found to occur on the cellulose surface at a high irradiation dose while at a low irradiation dose, the cellulose may undergo cross-linking(Takacs et al., 1999).
The effects of EB irradiation on the surface of cellulosic fiber can be explained using two different approaches. One is to investigate the surface morphology using scanning electron microscopy( SEM) and atomic force microscopy(AFM) and the other is to investigate the chemical characteristics of fiber surface using X-ray photoelectron spectroscopy(XPS), and Fourier transform infrared( FTIR) spectroscopy for chemical analysis(Koljonen et al., 2003; Oh et al., 2005). A combination of chemical and morphological information should yield better insight into the complex and functionally designed cellulose fiber surfaces.
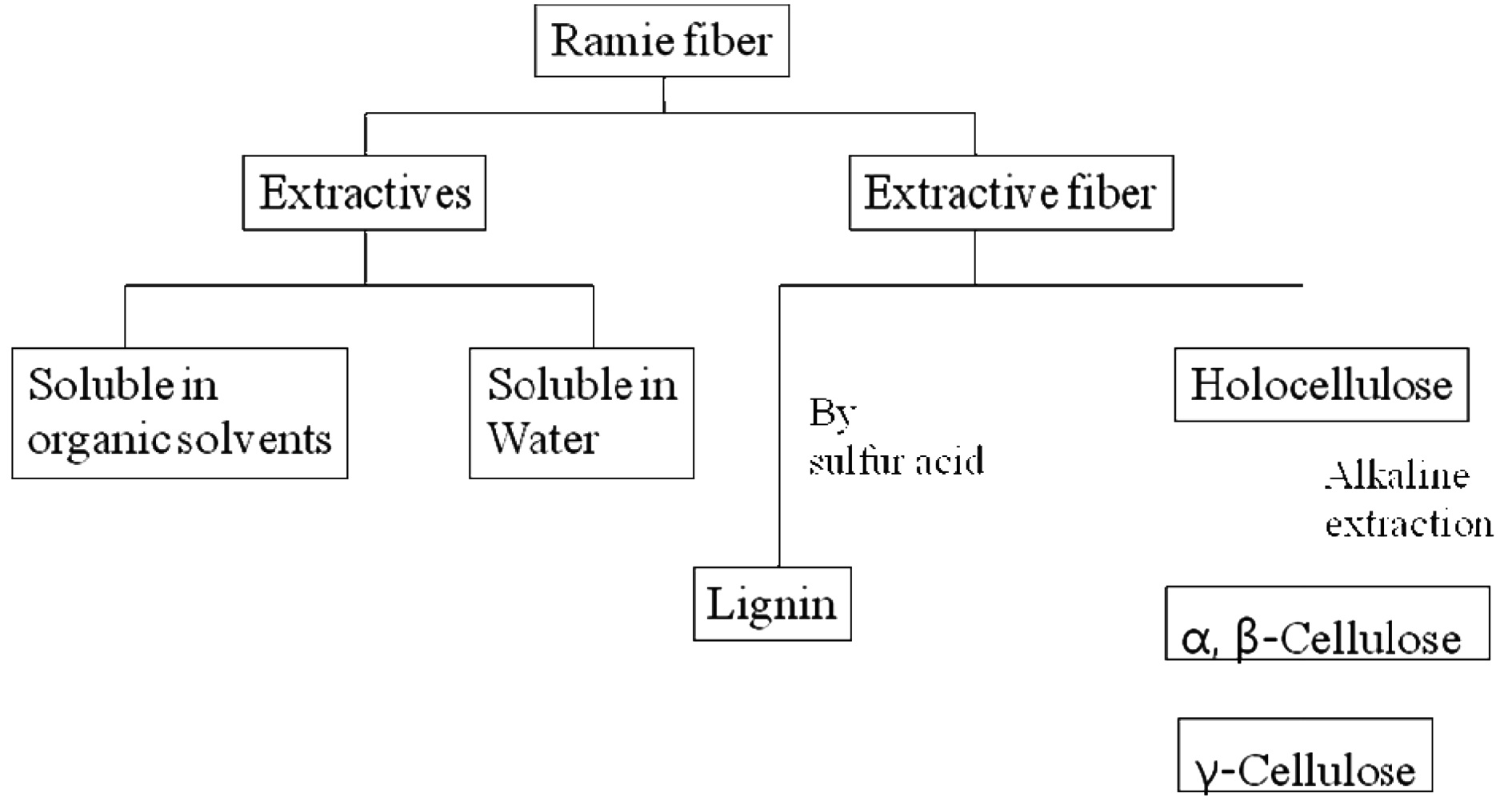
The present study was undertaken with the aim of elucidating the chemical and morphological surface changes induced by EB irradiation of ramie fibers using chemical component analysis, XPS, and SEM. The surface chemical changes were compared to the chemical components, which were analyzed according to TAPPI standards for alcohol-benzene soluble extraction and sulfuric acidinsoluble lignin, holocellulose and α, β-cellulose extraction with alkaline solution. The relationship between the chemical components or the surface chemical property and the complex structure was investigated using EB irradiation effects. The chemical and morphological studies of fibers by EB irradiation could provide vital information on the level of interfacial adhesion that would exist between the fiber and polymer matrix of biocomposite.
Ramie(
Ramie fibers were placed in a polyethylene bag and irradiated by an electron beam. The accelerator ELV-4 from eb-TECH Co. Ltd.(Daejeon, Korea) provided energy of 1.0MeV. The beam current was 1.8 mA and the beam had a transport velocity of 25 m/min. The dimensions of the beam extraction window were 980×980 mm. Electron beam irradiation doses of 1, 3, 5, and 10 were implemented, and a raw sample was used as a standard for comparison.
2.3. Chemical composition analysis
A standardized sampling procedure is provided in TAPPI Standard T257 cm-02, 2002. Ramie fibers were milled and used as
samples, which were larger than 40 mesh and smaller than 60 mesh. The procedure for chemical analysis of ramie fibers was conducted in accordance with TAPPI T264 cm-97, 1997. To prepare a sample for lignin and cellulose analysis, ethanol-benzene soluble extractives were investigated and removed by soaking in a solvent for 6h to remove the extra waxes, fats, and alcohol-soluble resins. After extraction with ethanol-benzene, the holocellulose, α and β-celluloses, and lignin contents were measured from the extractive-free fiber according to the scheme in Fig. 1. The contents of α and β-celluloses and lignin were determined through triplicate performance of methods TAPPI 203 cm-99 and T222 cm-02, 2002, and the average was recorded.
2.4. Surface chemical property by XPS analysis
XPS analysis was used to determine the surface chemical composition changes of the ramie fibers after EB irradiation. XPS measurements were performed with a Thermo MultiLab 2000 electron spectrometer using a monochromated Al Kα source operated at 300W. Survey scans were taken with a 1.0 eV step and a 50 eV analyzer pass energy, while the high resolution regional spectra were recorded at a 0.1 eV step and a 20 eV pass energy. Measurements were taken at three different spots on each sample in order to calculate an average of the heterogeneous surfaces.
2.5. Scanning electron microscopy
SEM(JEOL JSM-6390) was used to observe the surface morphologies of the un-irradiated ramie fibers and those irradiated at different EB doses. The acceleration voltage was 20 kV, and the samples were coated with Pt using a vacuum sputter coater.
3.1. Chemical component analysis
The properties of natural fibers depend on their source, nature, and storage. These properties, is turn, affect the mechanical properties of the biocomposite. The ramie fibers were composed of
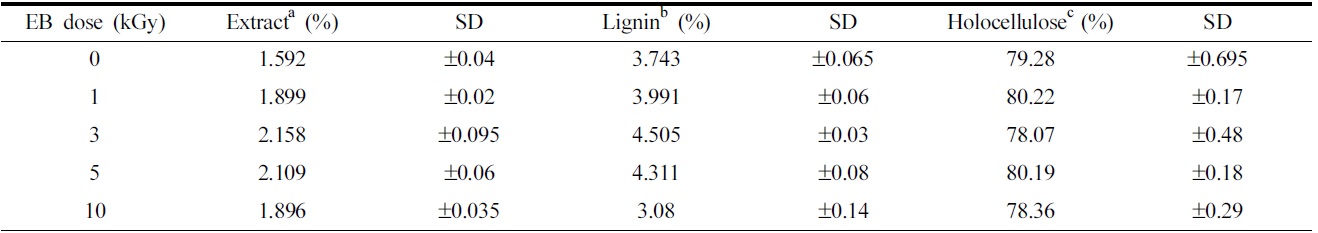
[Table 1.] The chemical compositions of ramie fibers irradiated with different EB doses.
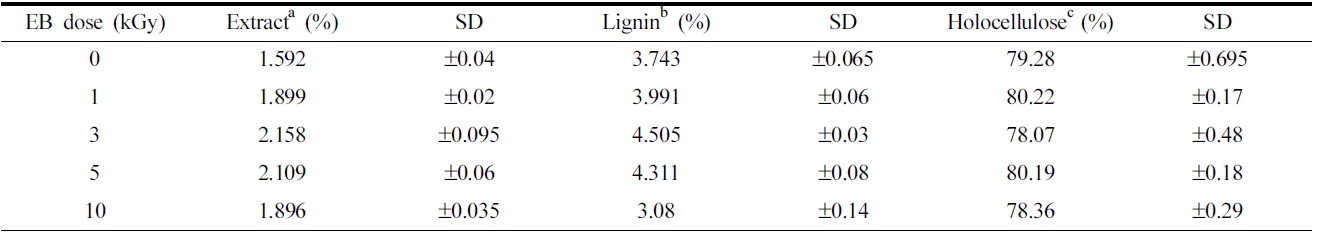
The chemical compositions of ramie fibers irradiated with different EB doses.
79.28% cellulose, 3.7% lignin, and 1.6% extractions, according to the results of chemical component analysis. Cellulose forms the main component of natural cellulosic fibers and consists of α-cellulose, β-cellulose, and γ-cellulose, which together make up holocellulose. The ramie fibers grown in Hansan, Korea, consist of 55.27% α-cellulose, 3.27% β-cellulose, and 20.74% γ-cellulose. Of all the celluloses, α-cellulose primarily determines the tensile strength of the fiber(Belgacem et al., 1995; Nevell & Zeronian, 1985).
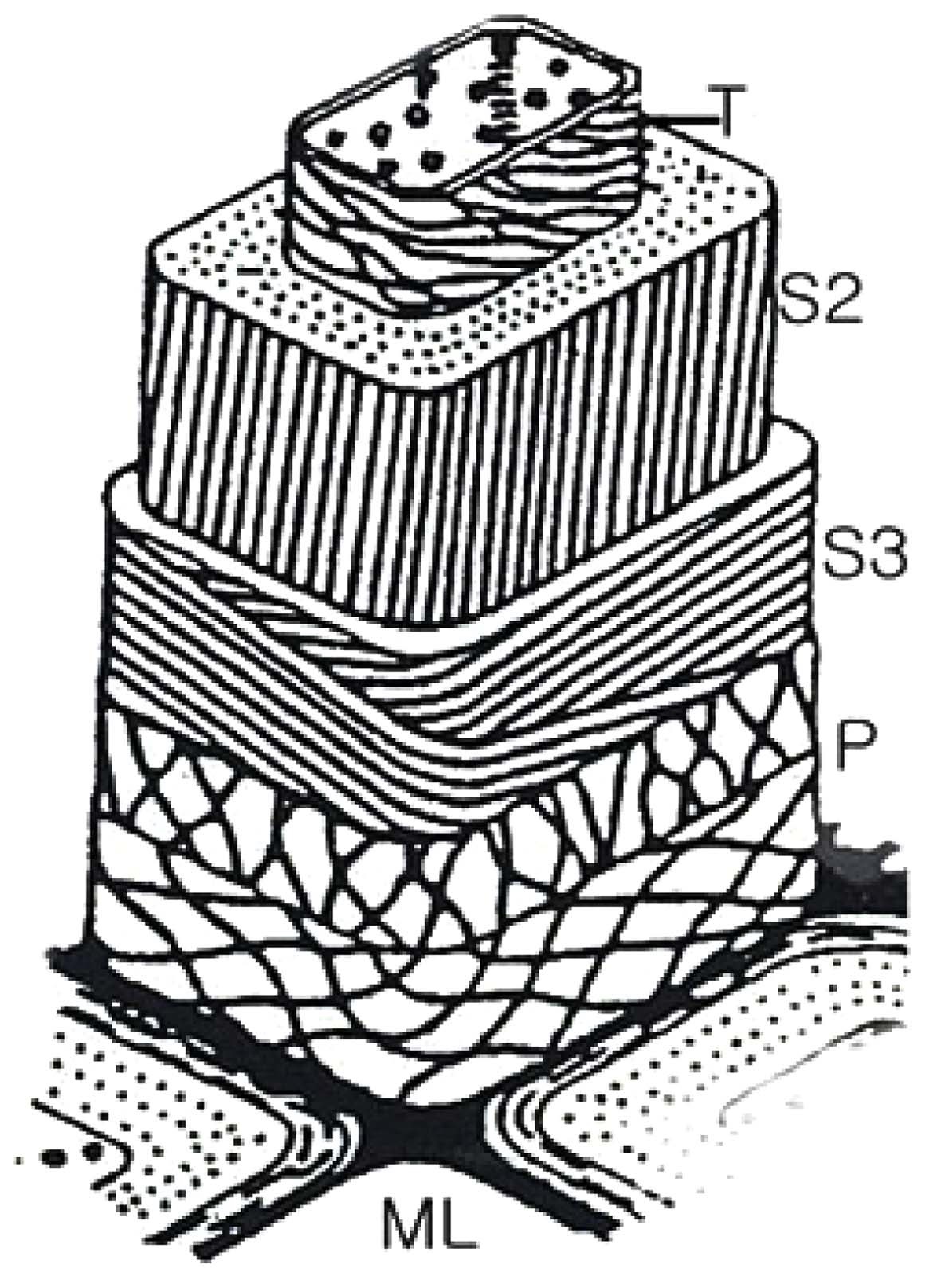
Changes in the chemical component, such as alcohol-benzene extracts, lignin, and cellulose in ramie fibers at the different EB irradiation doses are shown in Table 1. It has been shown that the amount of alcohol-benzene extract increases gradually with an increase in EB irradiation and reaching a maximum at an EB dose of 3 kGy, and decreases at 10 kGy. The walls of cellulosic fiber are constructed of four distinct layers: the middle lamella(ML) and the primary, secondary, and tertiary cell walls. Fig. 2 is the scheme of the morphological architecture of ramie fiber(Klemm et al., 1998). The exterior of the layer, the ML, contains mainly impurities and alcohol-benzene extractives, such as pectin and proteinaceous material. Adjacent to the ML, the primary wall(P) layer consists of 50% cellulose, as well as lignin and extracts, such as waxy, pectinaceous, and proteinaceous materials. In the untreated fibers, the impurities are completely attached to ML and P layer of the ramie fiber(Nevell & Zeronian, 1985). The impurities between ML and P layer were not dissolved well in the alcohol-benzene solution, which has a soluble content of 1.59%. A slight increase in the alcohol- benzene soluble content was observed when the ramie fibers were irradiated, and then the impurity content decreased at 10 kGy. As shown in Table 1, the content of lignin, one of the main components of cellulose, increased slightly upon EB irradiation up to 3 kGy. However, a large decrease in lignin content was observed at 10 kGy. These changes in lignin content could also be caused by a reduction in adhesion of lignin by the low dose and the removal of the cell walls, ML, and P layer due to the EB irradiation at 10 kGy, similar to the results of the alcohol-benzene extracts. The lignin
existing between the ML and P layer was removed with the removal of the ML and P layer by EB irradiation, leading to a decrease in the lignin content of the fibers of up to 3.08% at 10 kGy.
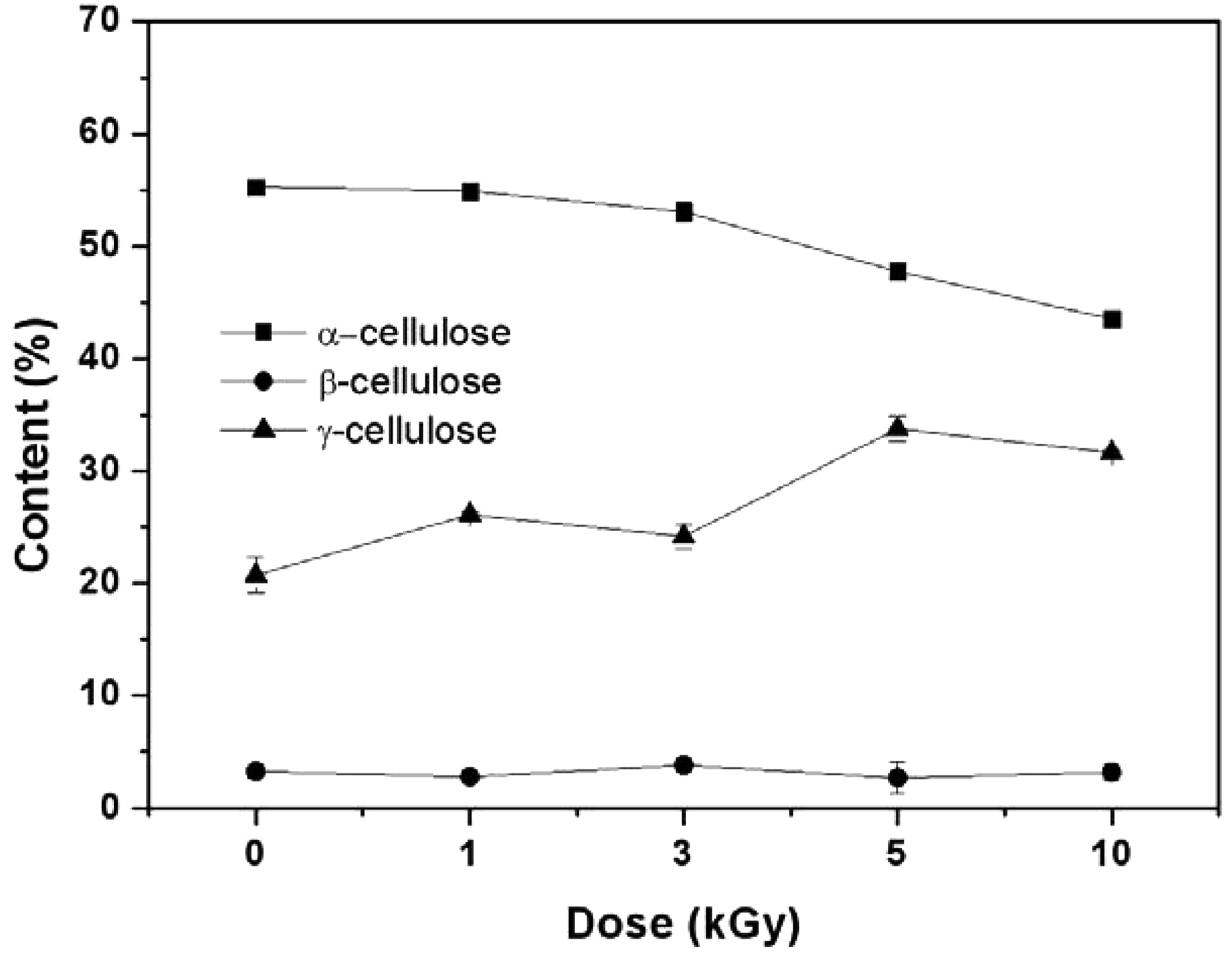
As seen in Table 1, no significant difference in the total holocellulose content was found as a result of EB irradiation, signifying that the cellulose component cannot be removed or transformed to another component through EB irradiation. Holocellulose consists of α-cellulose, β-cellulose and γ-cellulose. The content of α-cellulose was found to decrease while that of γ-cellulose increased with an increase in EB irradiation dose, as shown in Fig. 3. It means that the physical properties of fibers were transformed(Choi et al., 2009). In general, α-cellulose indicates higher-molecularweight cellulose and β and γ-cellulose indicates lower-molecular weight cellulose by degradation. The chain cutting of α-cellulose by EB irradiation results in a transformation into γ-cellulose composed of minor units of cellulose polymers.
3.2. Surface chemical analysis
There are two alternative XPS method for lignin analysis. One is based on determination of oxygen-to carbon atomic ratios, which are different for cellulose and lignin. In the other method, the carbon high-resolution C 1s spectrum is deconvoluted and the lignin content is calculated from the relative concentration of the C-C component. The C-C component in the C 1s spectrum, originating from carbon atoms that have no oxygen neighbors is due to lignin only, since C-C bonds are not present in chemically pure cellulose(Johansson, 2002).
XPS spectra were obtained for the un-irradiated and EB-irradiated ramie fibers. Cellulose is an organic compound with the formula(C6H10O5)n composed of the elements carbon, hydrogen, and oxygen.
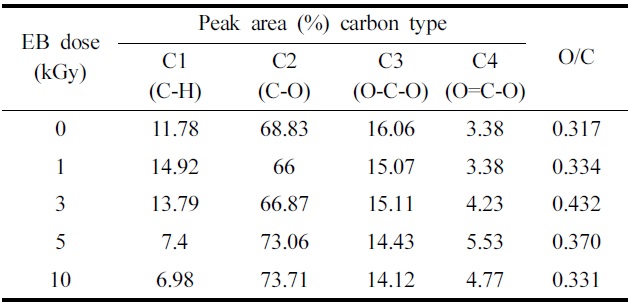
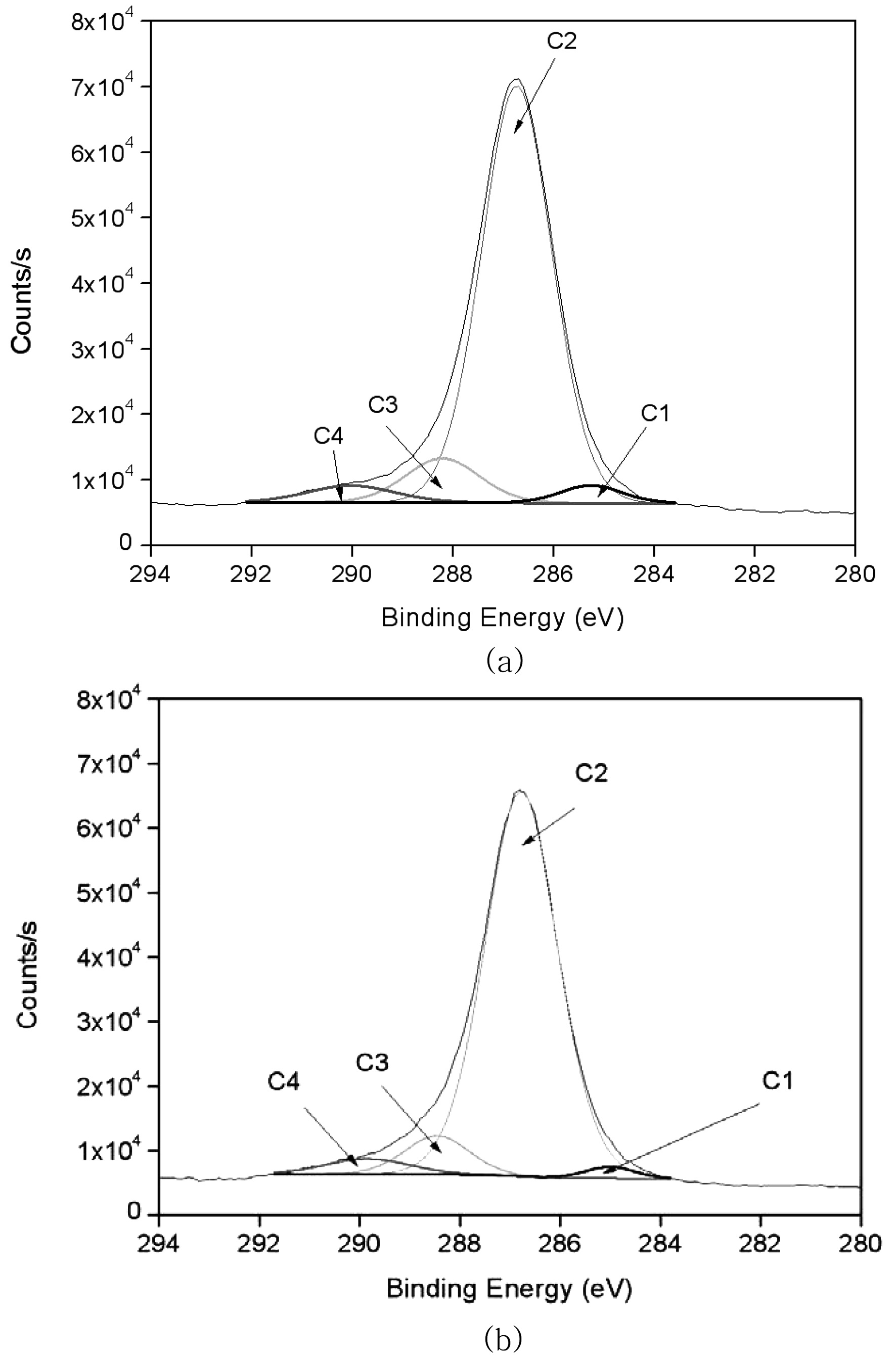
Table 2 summarizes the binding energies of the functional groups observed from carbon 1s spectra. The carbon 1s spectra were deconvoluted into four categories, which are shown in Fig. 4(a) and (b) are the representative spectrum of the un-irradiated and 10 kGy irradiated ramie fiber. Four carbon bond categories were assigned: C1 carbons bonded to hydrogen (C-H), C2 carbons bonded to one oxygen atom (C-O), C3 carbones from carbonyl groups (O-C-O), and C4 carbons from carboxyl group (O-C=O) (Juan et al., 2005). The C1 peak corresponds to non-oxidized alkane-type carbon atoms and originates from impurities like lignin, extractive substances, and fatty acids. Some of the impurities were obtained in the alcohol-benzene solution (Belgacem et al., 1995). In the case of XPS analysis, the surface impurity content was analyzed according to the C1 components (Laine et al., 1994). In this study, we presume that the lignin and alcohol-benzene extract contents correspond with the C1 component and O/C ratio. Table 2 shows a decrease in the C1 component from 3 kGy to 10 kGy. EB irradiation reduced the relative amount of C1 and increased the relative amount of C4 component. This suggests that the EB irradiation reduces the lignin(Koljonen et al., 2003). These results
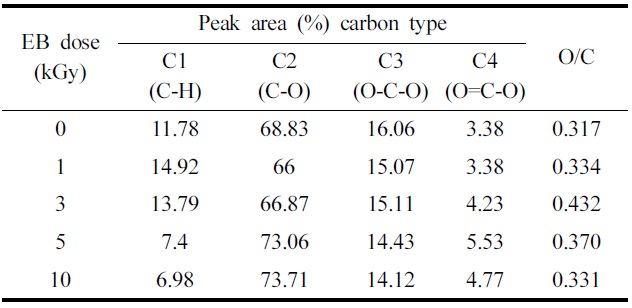
The effect of fiber surface chemical modification at various EB irradiation doses in ramie fiber.
indicate that the impurities were reduced from 3 kGy to 10 kGy. This can be explained by the cellular structure of natural fibers. The C1 component decreases with the removal of the exterior layer and ML and P layer-attached impurities by EB irradiation. Choi et al.(2008) reported that the surface morphology of cellulose fiber was changed by EB irradiation resulting from the removal of pectin, waxy and P layer at 10 kGy.
The carbon 1s spectrum was deconvoluted to determine the relative carbon-to-oxygen content. For charge compensation, the C-C binding peak was set to a binding energy of 285 eV to compare the
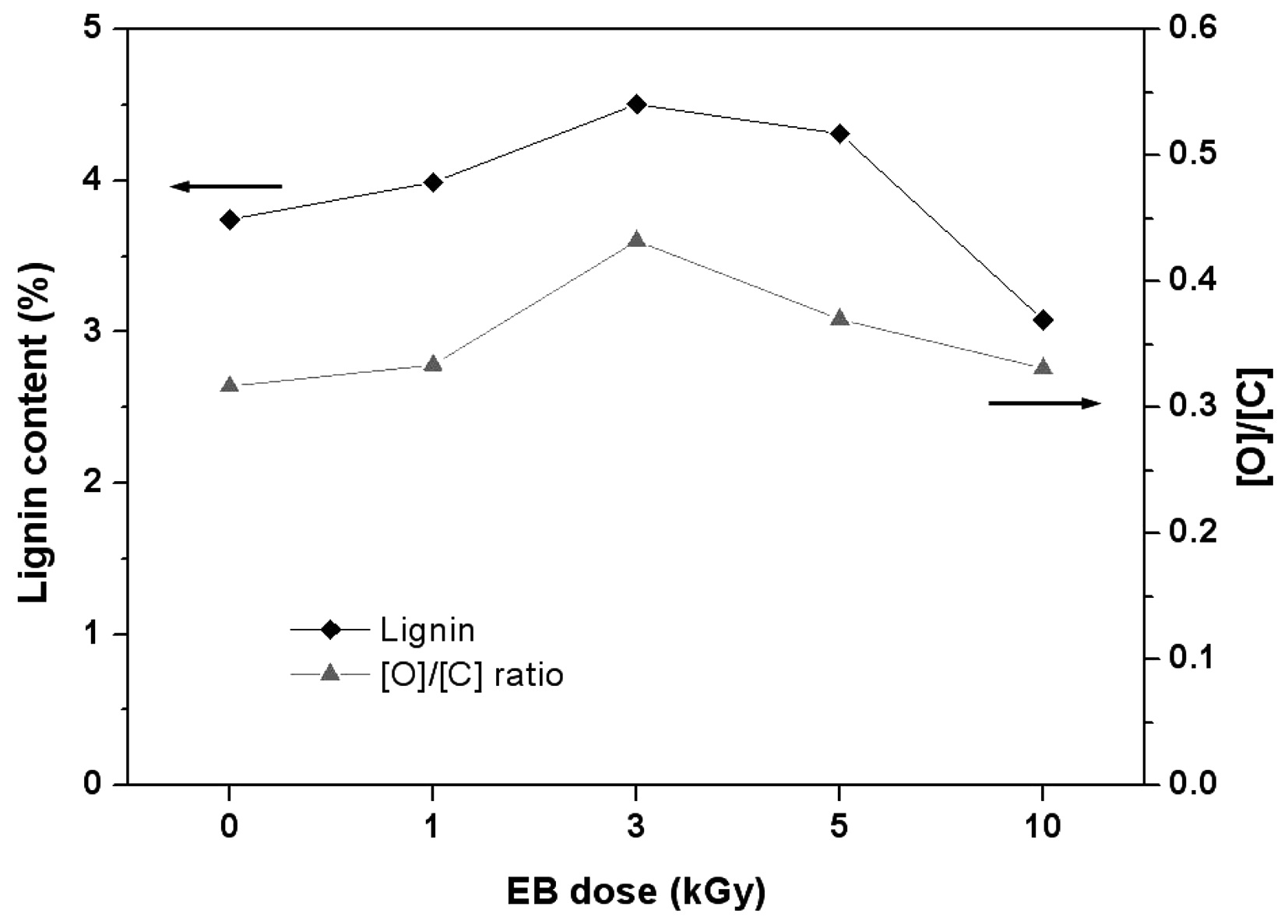
changes occurring in the O1s peak(Fig. 5). Table 2 also shows the atomic ratio of oxygen to carbon (O/C) on the surface of the ramie fibers irradiated with EB irradiation. Cellulose, hemicelluloses, and pectin have O/C ratios of 0.83, lignin has a ratio of about 0.35, and other extracts have even lower ratios(Leena-Sisko et al., 1999). Because the O/C ratio of ramie fiber is much lower than the expected value, less than 0.83, the surface must have contained a greater proportion of lignin and other extracts(Sgriccia et al., 2008). As aforementioned, this was due to the exterior walls of the fiber cells. The O/C ratio of the ramie fiber showed a maximum value at 3 kGy. This can be explained by the multi-layer structure. The secondary wall(S layer) under the P layer is composed of an outer S1 layer and inner S2 layer and innermost S3 layers. These three layers, the largest proportions of cellulose, are bound by lignin. From the O/C ratio results, we presume that the EB irradiation removed the surface layer of the ramie fiber partly, including the ML and P layer. Due to the removal of the ML and P layer, the O/C ratio was high at the low dose, 3 kGy. It has been shown that XPS can be used to evaluate the surface lignin content in ramie fibers irradiated with EB. Fig. 6 shows similar trends in the lignin content from the chemical component analysis and the O/C ratio obtained from the
surface chemical analysis.
3.3. Scanning electron microscopy
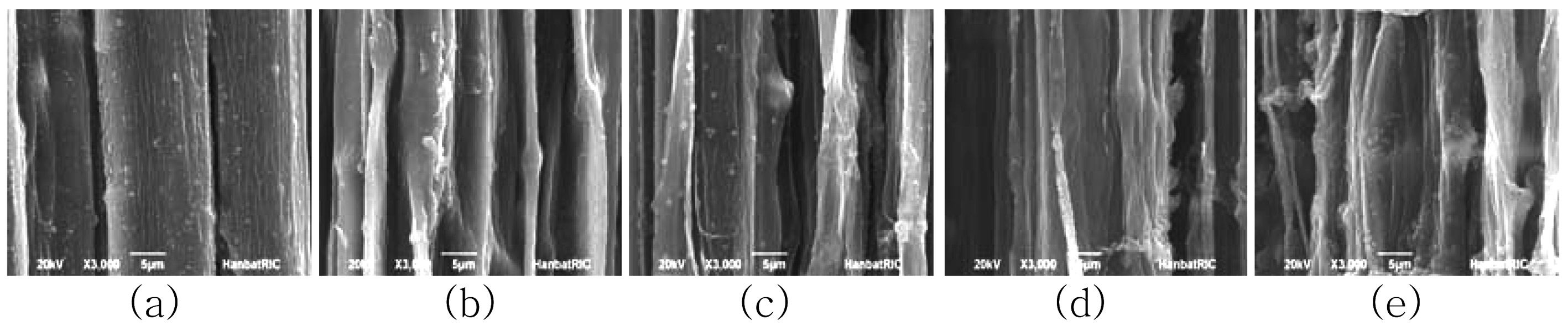
Scanning electron microscopy is an excellent technique for examining the surface morphology of fibers. It is expected that the surface morphology of the un-irradiated fiber will differ from that of the irradiated fibers, particularly in terms of roughness. Studies of fiber surface topography may provide vital information about the level of interfacial adhesion between fibers and the matrix when used later as reinforcement in a composite material. If the cellulose fiber has a rough surface, its adhesion property in the biocomposite will increase. Fig. 7 shows SEM images observed in the longitudinal directions for ramie fibers irradiated with different EB doses. The SEM image of the un-irradiated fiber shows a surface covered with weak boundary components, which act to smooth the surface. This is evidenced by the chemical analysis. The SEM images show the inter-fibrillar structure etched by EB irradiation. The ML and the P layer are located on the exterior of the fiber wall and contain abundant pectin, wax, and lignin. This is confirmed by the chemical component analysis results, which show a high concentration of alcohol-benzene extract and lignin. The XPS results also show high C1 components, which mean numerous impurities. At 10 kGy, the surface structure of the ramie fiber shows highly aligned and distinctive striations in a longitudinal direction. These results indicate that EB irradiation removes the ML and P layer and exposes the inner layer. With an increase in EB irradiation dose, the SEM images show more distinct and clearer surfaces as a result of the removal of impurities and exterior layers with irregular phases. The distinct and clear surfaces mean that the ramie fibers have a high cellulosic component. In chemical component analysis, the extract and lignin contents decreased; however, the holocellulose content did not decrease with increasing EB dose. These results indicate the removal of the ML and P layer containing impurities.
Also, XPS results showed an increase in C1 components, indicating impurities at higher doses. This means that the surface layer was damaged, and the lignin existing between the cellulose fibrils was exposed on the cell surface during EB irradiation.
This study investigated the chemical and morphological surface changes of ramie fibers induced by EB irradiation using chemical component analysis, XPS, and SEM. The results indicate that the surface of ramie fibers can be changed through EB irradiation. The chemical components and the surface chemical changes of the ramie fibers were generated by surface morphological changes.
Ramie fibers irradiated with different EB doses were analyzed using chemical component analysis and XPS. According to the chemical component analysis, with increasing irradiation doses up to 3 kGy, the extract and lignin contents increased and then decreased at doses greater than 10 kGy. These results correspond with the results of XPS at low doses of 1-10 kGy. The C1 peak and the O/C ratio indicating lignin and extractives display a similar trend with chemical component analysis.
The surface morphology of the untreated fiber was also investigated. The SEM image of the untreated fiber shows a surface covered with weak boundary components and primarily consisting of abundant pectin, wax, and lignin. The SEM images show the interfibrillar material at low doses of EB irradiation up to 5 kGy. After irradiation with doses greater than 10 kGy, the surface structure of the ramie fiber is highly aligned, with distinctive striations in a longitudinal direction. These results indicate that 10 kGy EB irradiation removed the ML and P layer while exposed the inner layer. The more distinct and clearer surface indicates that some of the impurities and exterior layers having irregular phases were removed with EB irradiation.