Expanded graphite (EG) is a well-known carbon material that is usually produced from various graphite intercalation compounds (GICs). The process of GIC exfoliation may be undertaken using chemical, electrochemical, and thermal methods. EG maintains layered structures similar to natural flake graphite, but it has differences in the distances between the graphite layers. As a new carbon material, EG has been used in many fields, such as gaskets, seals, batteries, substratum for adsorption, etc. Among these applications, its potential as an adsorbent for heavy oil has attracted significant attention due to the surprisingly high adsorption capacity of EG for various oils [1-7].
Toyoda and Inagaki [8] studied the adsorption behaviors of exfoliated graphites using four types of heavy oils. The results indicated that the maximum adsorption capacity of 1 g of exfoliated graphite was as high as 86 g of A-grade heavy oil or 76 g of crude oil. Zheng et al. [9] investigated the adsorption capacity of exfoliated graphite for oil-sorption in and among worm-like particles. Their results demonstrated that the adsorption capacities of the pores in and among the worm-like particles increased with increases in the exfoliation volume.
In this study, EG was prepared using a new drying process for oil-adsorbent materials, and its morphology, expansion volume, and oil absorption capacity are investigated.
The graphite used in this study was supplied by GRAF Guard™ (>297 μm, flake graphite). Oleum (25% SO3) was used as an intercalant, and n-dodecane was purchased from Sigma (Korea).
The GICs were prepared using the direct reaction of graphite with oleum gas in atmospheric conditions. In order to intercalate SO3 in the graphite layers, SO3 gas was vaporized through heating the oleum at 120℃ and passing it through an upper flask filled with graphites. The GICs were dried at 90℃ for 8 h in order to remove the SO3 molecules condensed on the graphite surfaces. The EG was obtained through heat treatment of the dried graphite particles at 900℃ for 1 min.
2.3. Characterization and measurements
The morphology of the EG was examined using a scanning electron microscopy (SEM; JXA 840A, JEOL, Japan). The expansion volume was calculated from the EG volumes before and after the heat treatment. The oil adsorption tests were conducted
where
Fig. 1 presents a SEM image of the EG. As seen from this image, there were numerous carbon layers with large interlayer distances, which were formed as a result of the heat treatment of the GICs at a sufficiently high temperature. Furthermore, the EG had a loose and porous vermicular structure. The structure of the EG appeared to have parallel boards and it exhibited many pores with different sizes [10,11].
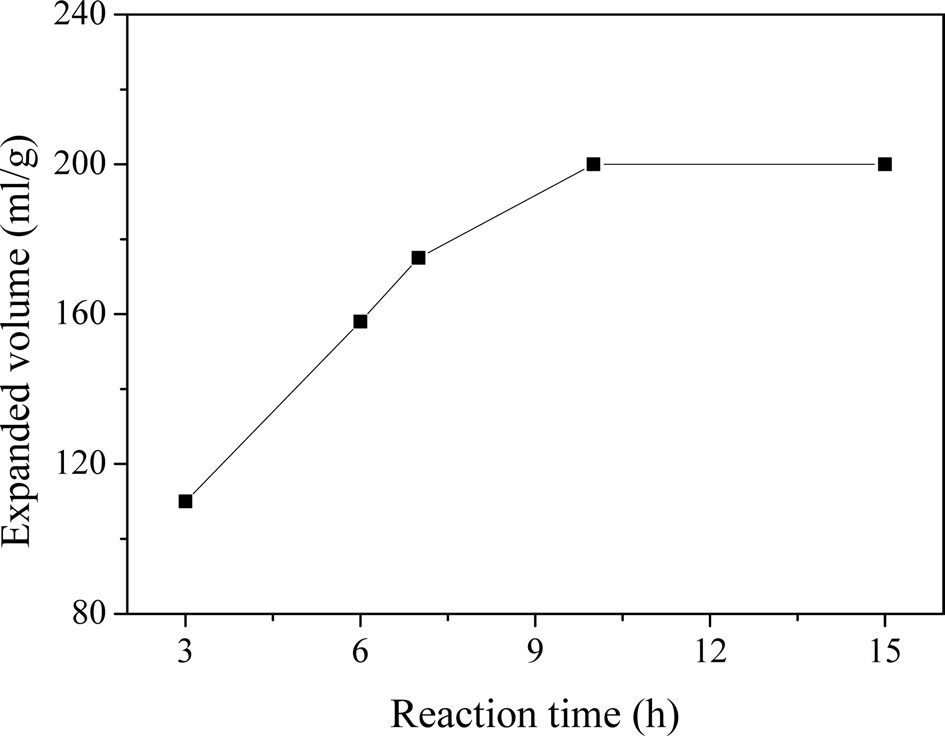
Fig. 2 presents the expanded volume of the EG as a function of the reaction time. The expanded volume of the EG increased significantly with an increasing reaction time up to 10 h. When the reaction time was increased, the graphite layers opened gradually due to the oxidizing agent, and the molecules of the inserting reagent entered the graphite layers, which resulted in the formation of a large amount of GICs. Thus, the expansion volume of the EG increased rapidly [12].
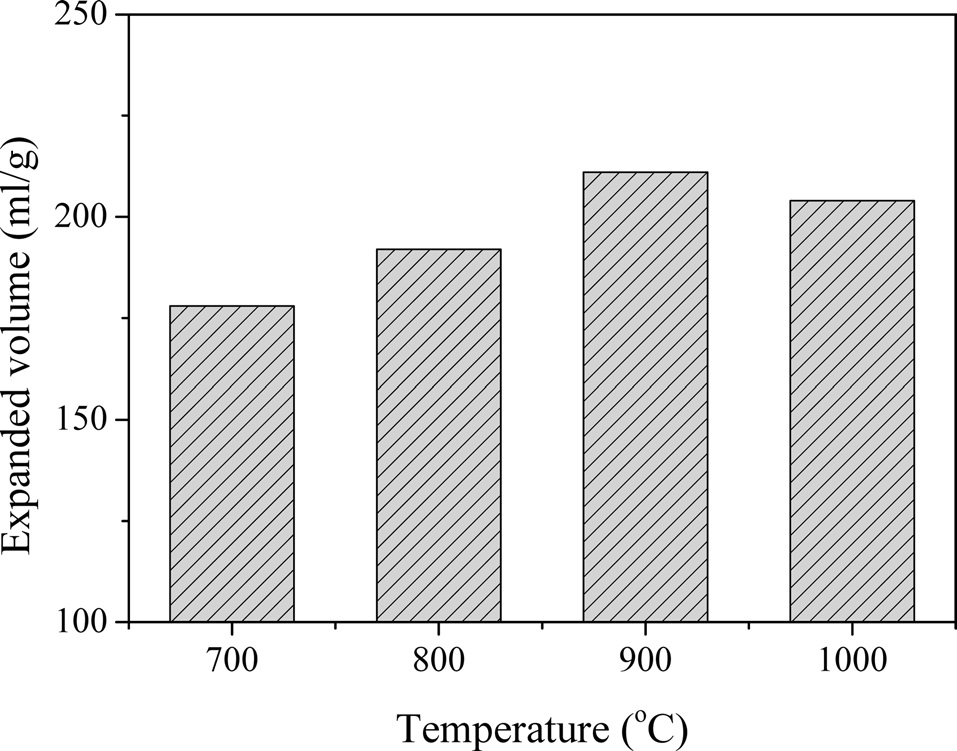
Fig. 3 presents the expanded volume of the EG as a function of the heat treatment temperature. When the heat treatment temperature increased, the expanded volume increased gradually with the maximum expanded volume being achieved at 900℃ and the expanded volume decreasing over 900℃. This can be attributed to the expanded GIC needing energy; however, some carbon began to combust over 900℃ [12].
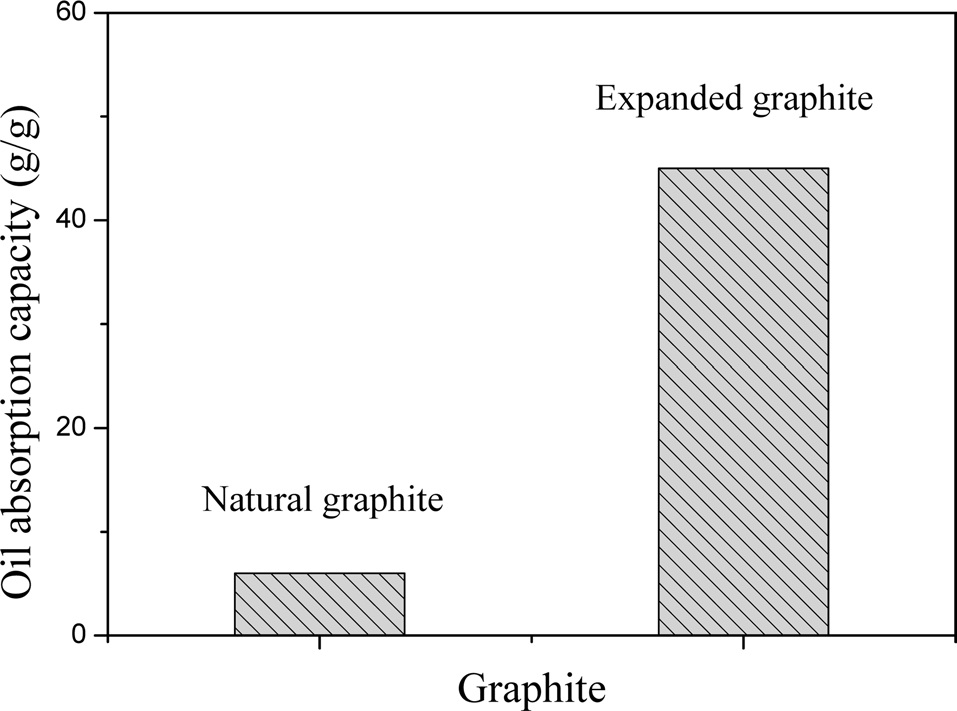
Fig. 4 shows the oil adsorption capacity of natural graphite and EG for n-dodecane. The oil adsorption tests indicated that the n-dodecane was absorbed rapidly by the EG. The oil adsorption capacity of EG (45 g of n-dodecane per 1 g of EG) was significantly higher than that of natural graphite. These results can be attributed to the EG having surface characteristics that consist of micropores and macropores, which induce the capillary phenomenon between the particles and pores on the surface of the EG and result in an increasing oil adsorption capacity [13,14].
In this work, EG was prepared using a drying process for potential application as an oil-adsorbent. The morphology,
expansion volume, and oil adsorption capacity of EG were investigated. As expected, the expanded volume of the EG increased with an increasing heat treatment temperature up to 900℃. The oil adsorption capacity of EG was 45 g of n-dodecane per 1 g of EG. The data reported in this study suggests that EG has excellent properties and is a promising candidate for oiladsorbent applications.