Microalgae are an important component of the aquatic environment (Elser et al., 2007), and are key players in primary productivity and biogeochemical cycles (Sarthou et al., 2005). They are rich in nutrients and organic compounds, and hence are used as aquaculture feeds, health supplements and alternative energy sources (Becker, 2007). Moreover, microalgae are a diverse assemblage of both autotrophs and heterotrophs, have substantial biomass, and are abundant in the marine ecosystem (Shi et al., 2011). They are especially useful as bioindicators of environmental changes, for both short and long-term environmental monitoring as well as ecotoxicology assessments (Franklin et al., 2002). Owing to the involvement of microalgae in the global cycling of toxic chemicals in the aquatic environment, monitoring the effects of chemicals, such as metals and endocrine disruptors, on microalgae is of considerable importance (Torres et al., 2008).
Algae-based bioassays are commonly employed in environmental risk assessments to assess the toxicity of metals, novel chemicals and other emerging contaminants and to establish regulatory guidelines (Stauber and Davies, 2000). Algal toxicity tests routinely use freshwater green algae (
In the present study, we quantified the sub-lethal effects of metals and endocrine-disrupting chemicals (EDCs) on the marine green alga
>
Test species and culture conditions
In this study, three metals (Cu, Ni, and Pb) and three EDCs (BPA, ES, and PCB) were selected as test toxicants. The concentrations of each were chosen based on EC50 values reported for other aquatic organisms (Millan de Kuhn et al., 2006; Soto-Jimenez et al., 2011). Accordingly, a range of concentrations of each chemical was prepared, as described below.
For Cu (as CuSO4; Cat. No. C1297, Sigma, St. Louis, MO, USA), the concentrations used were 0.5, 1.5, 3, 6, 12, 25, 75, 150, 500, and 750 mg/L. For Pb (as PbCl2; Cat. No. 268690, Sigma), the chosen concentrations were 0.5, 1.5, 5, 10, 25, 50, 100, 150, 250, 500 and 750 mg/L. For Ni (as NiCl2; Cat. No. 339350, Sigma), the concentrations used were 0.1, 0.5, 1, 5, 10, 50, 100, 250, and 500 mg/L. All test concentrations were higher than that added to the f/2 medium. For example, amongst the four test metals, only 0.0068 mg/L CuSO4 was added to the f/2 medium, and thus the medium-containing metals or EDCs contained negligible endpoint concentrations.
For BPA (Cat. No. A133027, Sigma), concentrations of 0.5, 1, 2.5, 7.5, 15, 25, 50, 75, and 100 mg/L were prepared from a stock solution. BPA was dissolved in 10% dimethyl sulfoxide. For ES (Cat. No. 36676, Sigma), the concentrations used were 0.001, 0.01, 0.1, 0.5, 1 and 10 mg/L. PCBs were prepared from Aroclor 1016 (Cat. No. 48701, Sigma) at 0.001, 0.01, 0.05, 0.1, 0.5, 1, 10, 20, and 50 mg/L. All dilutions were from standard stock solutions.
Fifty-milliliter aliquots of the algal culture, comprising cells in the exponential phase of growth, were transferred into sterile tubes. The chemicals, at the concentrations mentioned above, were transferred into test tubes in duplicate. The initial cell concentration was 5.5 ± 0.1 × 105/mL. and samples were withdrawn for cell counts and chlorophyll
Cells were enumerated using a hemocytometer (Marienfeld GmbH, Lauda, Germany). Cell counts were plotted against exposed time as log10 values. In addition, Chl
The median effective concentration (EC50) and the percentile growth inhibition were calculated as recommended in the Organisation for Economic Cooperation and Development (OECD) test guidelines (OECD, 2011). The 72-h EC50 values were estimated using a sigmoidal dose-response curve plotted in Origin 8.5 (MicroCal Software Inc., Northampton, MA, USA) based on the sigmoidal four parameter equation (Teisseyre and Mozrzymas, 2006): Log EC50 =
All data presented are the mean values of duplicate determinations. A one-way analysis of variance (ANOVA) with post hoc Student’s Newmann Keul’s test in Graphpad InStat (Graphpad Software, Inc., La Jolla, CA, USA) was used for comparisons between non-treated and treated cultures.
>
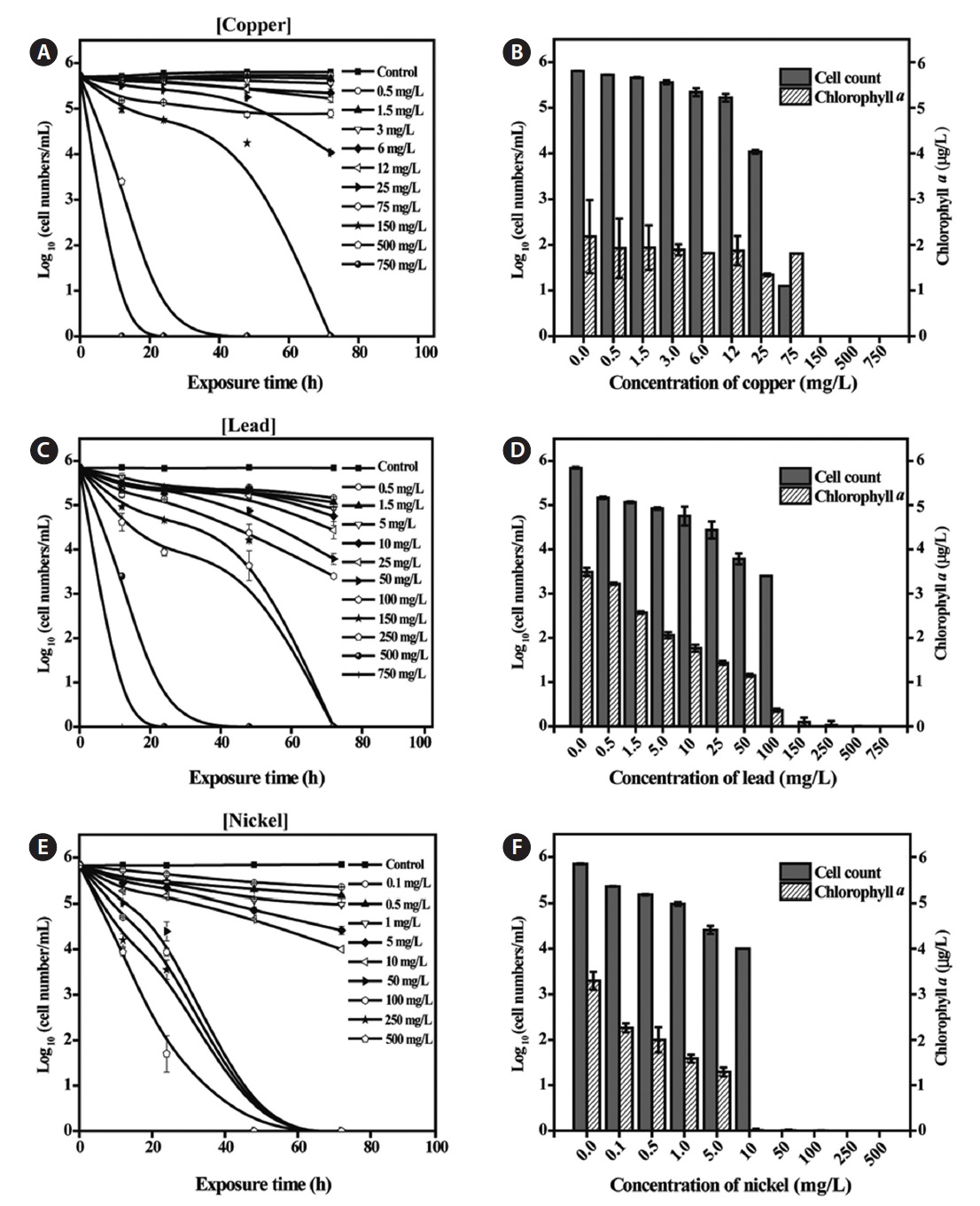
Toxicity of metals to Tetraselmis suecica
Exposure of
>
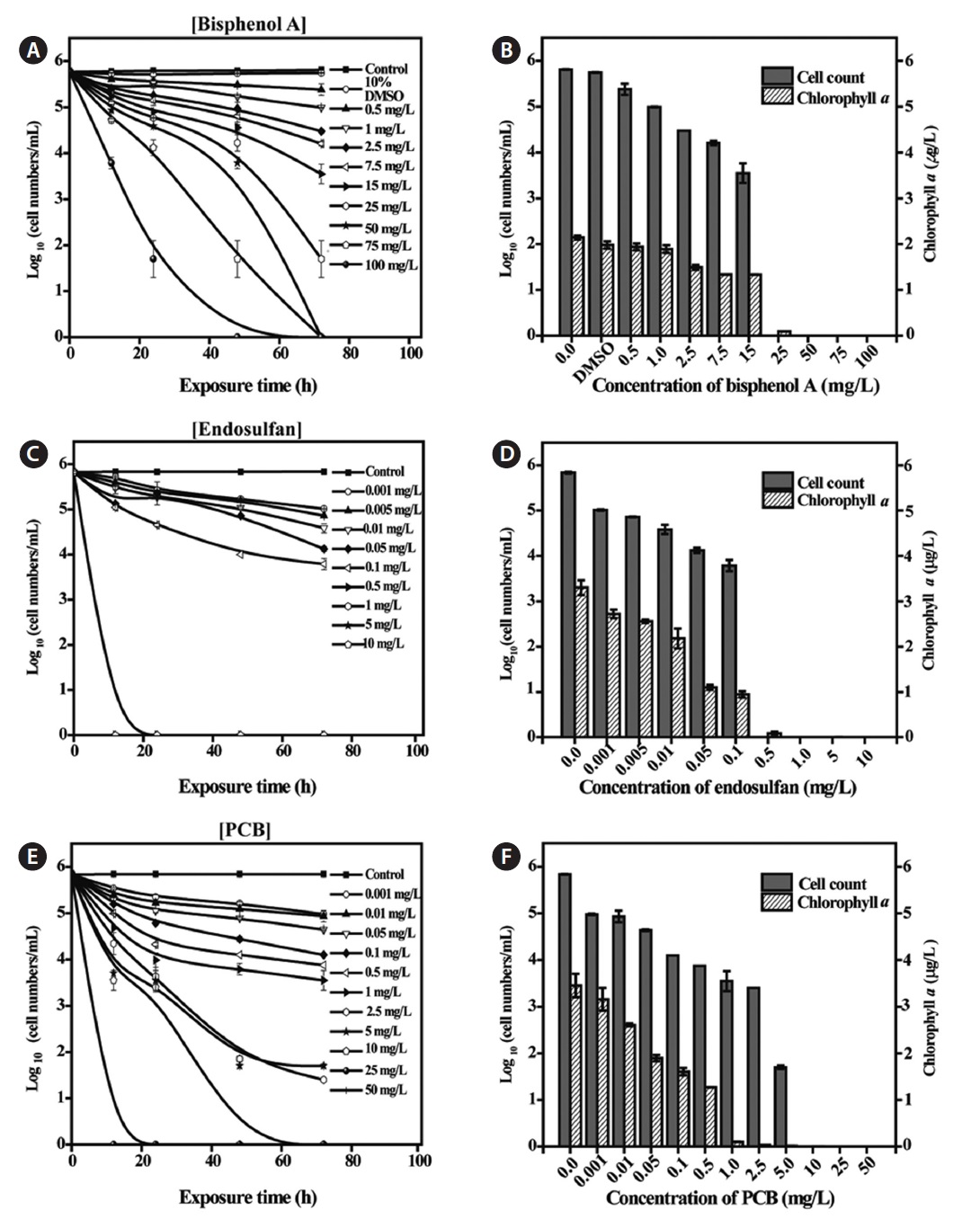
Toxicity of EDCs to Tetraselmis suecica
Additional toxicity tests for the three EDCs (BPA, ES, and PCB) were performed over a wide range of concentrations (Fig. 2). BPA was administered at concentrations of 0.5-100 mg/L.
>
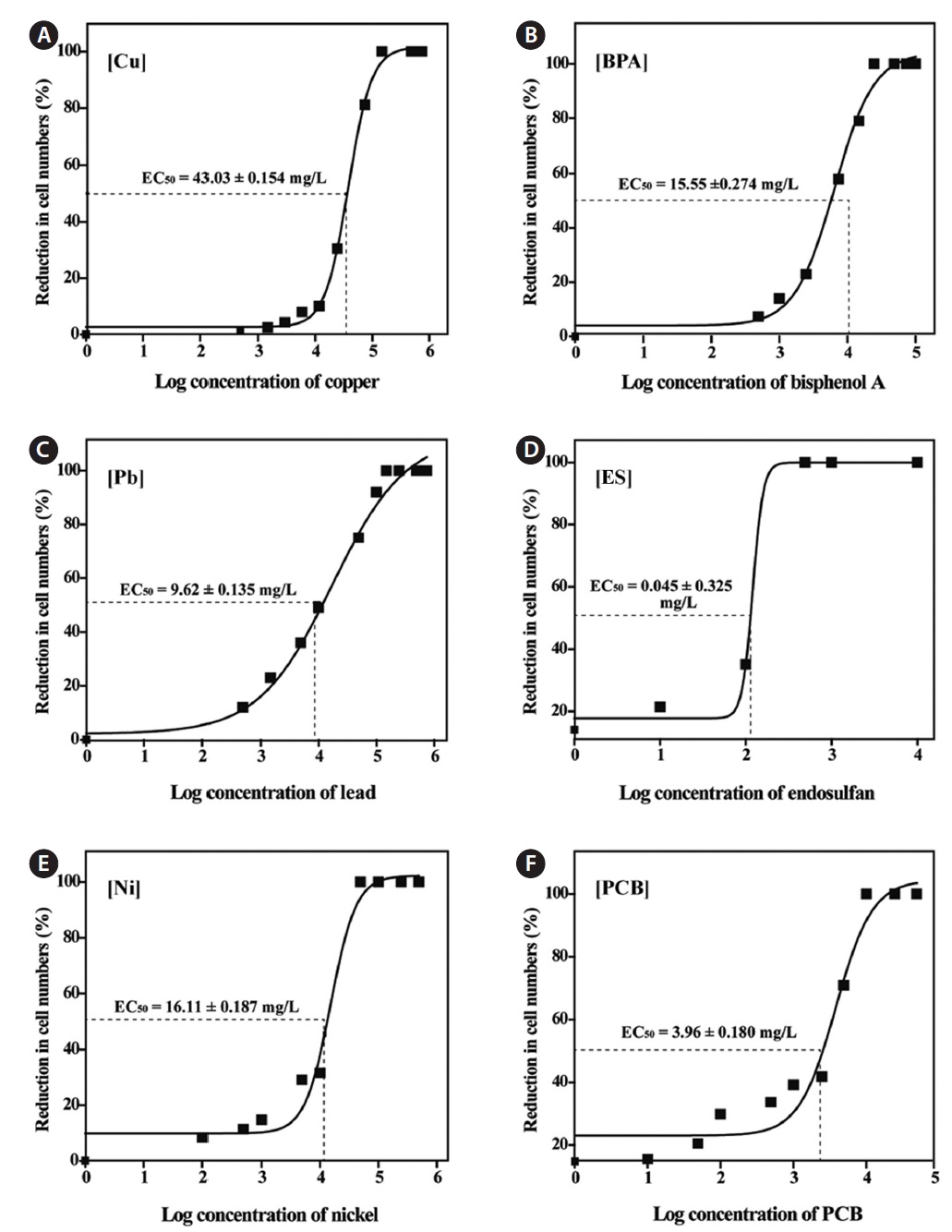
Dose-response curves and 72-h EC50 values
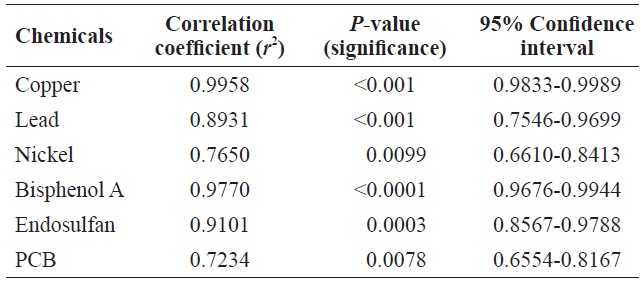
Table 1 shows the Pearson’s correlations between cell count and Chl
Toxicity assessments using marine species can be challenging compared with assessments using freshwater species. This is because the marine environment can have a more profound influence on toxicity evaluation than freshwater ecosystems. In addition, the higher ionic strength and buffering capacity of seawater can alter the bioavailability of discharged chemicals due to complex chemical reactions and the subsequent formation of by-products (Moffett and Zika, 1987). As noted previously, relatively few species can be described as “standard” for marine algae, although several guidelines have been published and some marine species have been recommended as model species (Nalewajko and Olaveson, 1998; Sverdrup et al., 2001). In this study, we present additional toxicity data for various metals and EDCs to the marine green alga
Discharge guidelines for metals in the marine environment are as follows: Cu, 0.5 mg/L; Pb and Ni, 0.1 mg/L (United States Environmental Protection Agency, 1996). Individual discharges of Cu, Pb, or Ni confined to the stipulated levels should have very little, if any, effects on cell counts or Chl
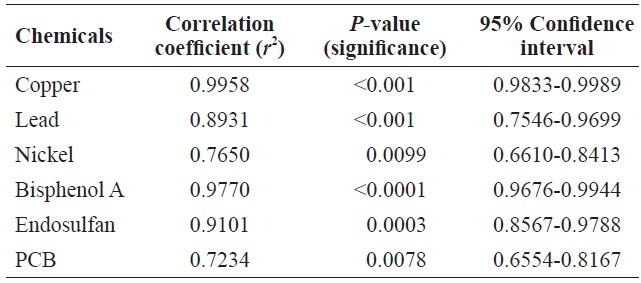
Pearson’s correlation between cell count and Chl a level in Tetraselmis suecica cells following exposure to toxic chemicals
[Table 2.] 72-h EC50 values of chemicals exposed to Tetraselmis suecica
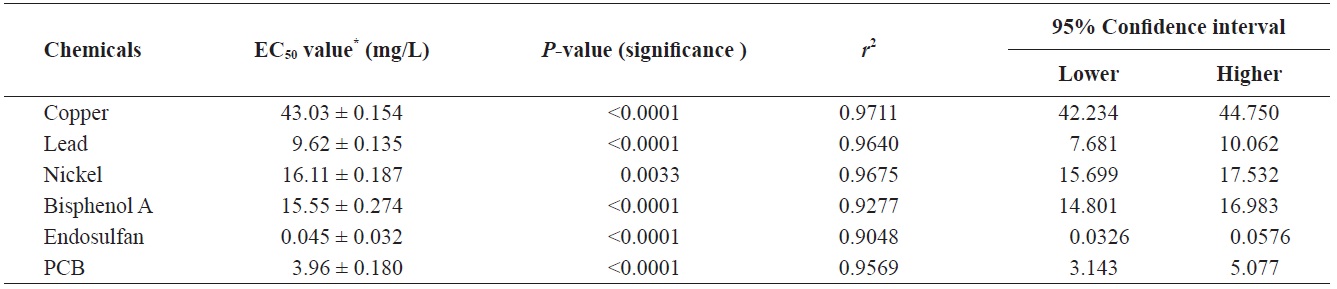
72-h EC50 values of chemicals exposed to Tetraselmis suecica
the 72-h EC50 value for Cu was 0.016 mg/L in
In addition, comparisons of available EC50 data revealed that our test species was generally more tolerant than other freshwater algae, including
To date, the available data regarding the toxicity of EDCs to algae is limited compared to metals. One reason for this is that algae do not have an endocrine system, and thus may show limited effects of exposure to EDCs. However, recent studies have shown that most EDCs, such as BPA, ES, PCB or metolachlor, do have toxic effects on algae (Liu et al., 2010; Ebenezer and Ki, 2012), in particular by damaging photo system II energy fluxes in chloroplasts (Perron and Juneau, 2011). In contrast, the data from this study indicated that
In conclusion,