Transuranic elements, such as Np, Pu, Am, and Cm, in spent nuclear fuel are important for fuel characterization [1,2], burnup credit [3,4,5,6], and the management of radioactive wastes [7] and are used for code validation through which the amount of nuclides produced or decayed during neutron irradiation is predicted. For the determination of 237Np in spent nuclear fuel samples, the selection of an appropriate tracer is required depending on the detection technique, such as alpha spectrometry or mass spectrometry, as 237Np has a very low specific activity owing to its long half life (2.14x106 y), which controls the sample amounts according to the detection method and the type of tracer. Generally, the determination of 237Np in environmental samples has been performed by alpha spectrometry, inductively coupled plasma mass spectrometry (ICP-MS), thermal ionization mass spectrometry, and neutron activation gamma spectrometry using 235Np (t1/2=396.1d, α) [8], 236Np (t1/2= 5000y, β) [9,10] and 239Np (t1/2=2.35d, β,
In this case, another method should be used as an alternative. Thus, an isotope dilution method for the determination of 237Np in a spent fuel sample using 239Np as a spike was previously developed in our laboratory, in which 237Np was detected by alpha spectrometry and 239Np by gamma spectrometry [12,13]. However, this method has a weak point showing a high measurement uncertainty owing to too low an alpha specific activity of 237Np and a high gamma specific activity of 239Np for a given sample amount, which causes too low an activity ratio of 237Np/239Np. This ratio is used as one of the terms in the equation related to the isotope dilution method.
In this work, inductively coupled plasma mass spectrometry (ICP-MS) was taken for the measurement of 237Np instead of alpha spetrometry to reduce measurement errors caused by the too low alpha activity of 237Np because ICP-MS has a higher sensitivity, lower measurement uncertainty, and more convenience in measurement compared to alpha spectrometry. This method was applied to PWR spent fuel samples and the 237Np contents were determined. The measured values were compared with the values calculated using the ORIGEN-2 code [14].
The standard solution of 243Am (North America Scientific Inc) was used for 239Np as a spike as 243Am enters a radioequilibrium state with 239Np after a certain time depending on the half lives of the two nuclides. The standard solution of 237Np for ICP-MS was obtained from the Damri company of France (CEA). An anion exchanger (AGMP- 1 x 8, 100-200 mesh size) was obtained from Bio-Rad Laboratories, USA. A disposable polyethylene column filled with an anion exchanger (7 mm id x 70 mm h) was used. Inorganic acids such as hydrochloric acid and nitric acid used for the sample treatments were the products of an extra pure grade from the Junsei Company, Japan and GR from Merck Company, Germany, respectively. Hydroxylaminehydrochloride and hydriodic acid used for the reduction of Pu and Np, and elution of Pu, respectively, were of GR grade from Merck Company. Radiation shielded inductively coupled plasma atomic emission spectroscopy (ICP-AES), used for the determination of uranium in the sample solutions, was an IRIS-HS model from Thermo Jarrell Ash, USA. The inductively coupled plasma mass spectrometer (ICP-MS) used for the determination of 237Np was a Finnigan Mat, Element model from Finnigan, Germany.
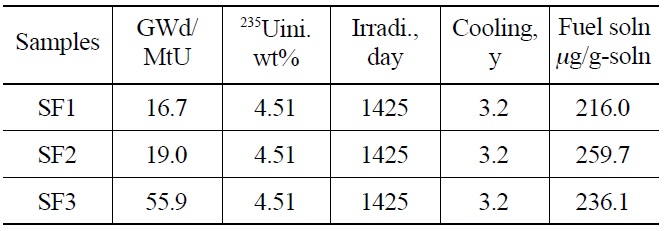
Three PWR spent fuel samples with burnups of 16.7- 55.9 GWd/MtU were taken from a nuclear power plant in Korea (Table 1). The initial concentration of 235U was 4.51wt% and the cooling time for the spent fuel samples was 3.2 years. A small piece of each specimen (~ 0.5g) was dissolved in (1+1) nitric acid under a reflux condenser in a chemical hot cell. A mother solution was diluted to ~ 0.2 μgU/mL, and an appropriate amount of the diluted solution was sent to a glove box using a pneumatic transfer. The uranium content in the diluted solutions was determined by a radiation-shielded ICP-AES followed by a neptunium separation.
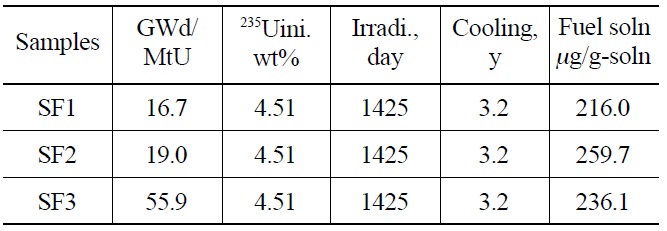
Spent Fuel History
2.3 Separation and Measurements
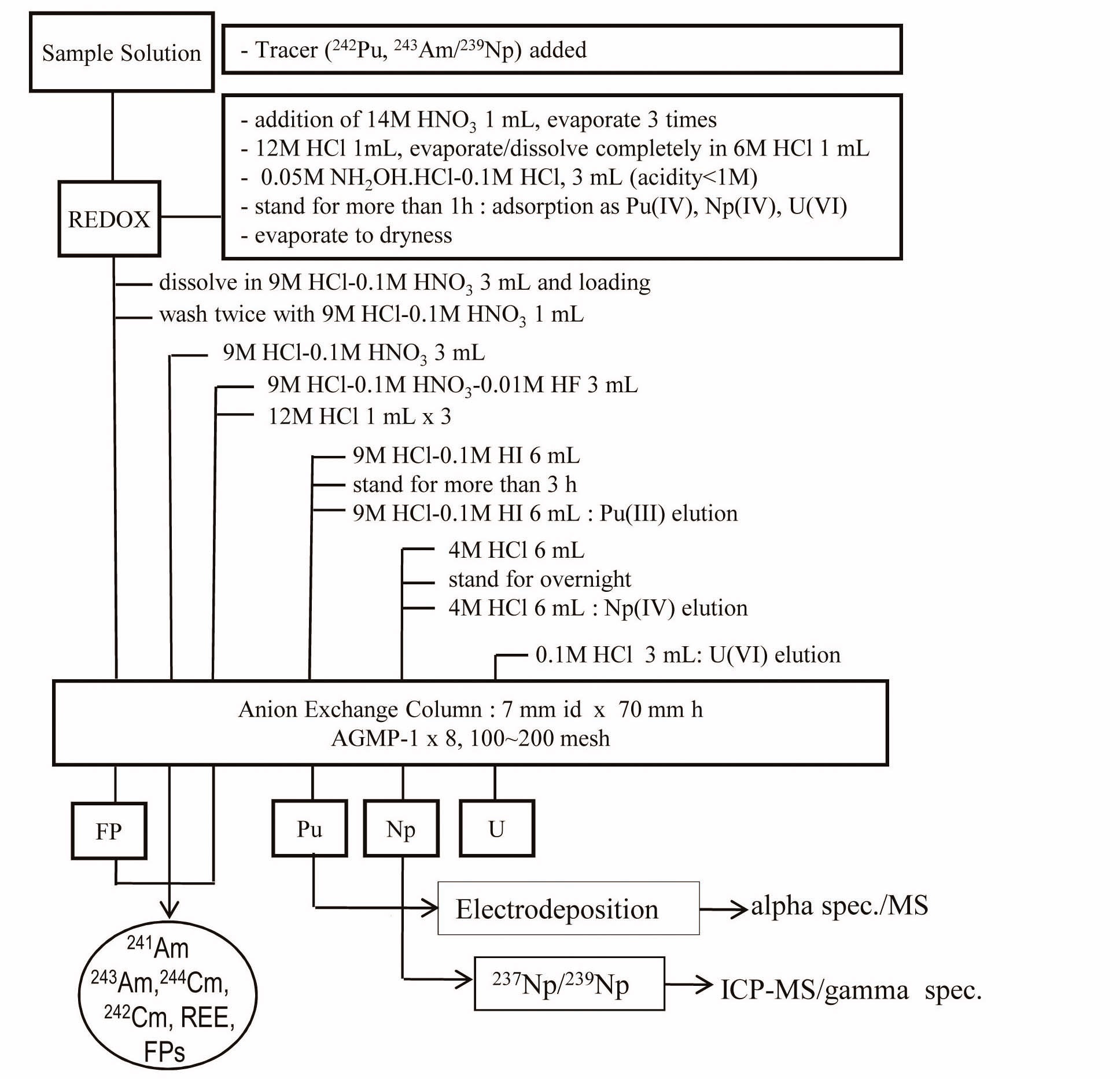
Two appropriate amounts of diluted sample solutions, equivalent to ~10 μgU each, were taken as the "sample" and "spiked sample." The solution for the "spiked sample" was spiked with 30 Bq of 239Np (243Am). The two sample solutions were treated with HNO3 and HCl on a hot plate two or three times repeatedly. The residue was dissolved by 3 mL of 0.05 M NH2OH.HCl-0.1M HCl and left overnight to obtain Pu(III) and Np(IV). Finally, prior to the separation, the sample solutions were made in a medium of 9M HCl-0.1M HNO3. In this step, Pu(III) is oxidized to Pu(IV). Two anion exchange columns were prepared for the "sample" and "spiked sample." The treated sample solutions were loaded onto each column. The following steps were conducted according to the procedure shown in Fig. 1 [15]. The Np fraction was collected by an elution of 12 mL of 4 M HCl after the elution of Pu with 12 mL of 9 M HCl-0.1 M HI. The collected fraction of Np was evaporated on a hot plate, and the medium was changed into nitrate form using c-HNO3. The gamma activities of 239Np were measured as soon as possible after separation, and the 237Np amounts were measured by ICP-MS. Finally, the 237Np contents in the sample solutions were obtained through an isotope dilution equation using the ratios of 237Np/239Np in the “sample” and “spiked sample,” respectively.
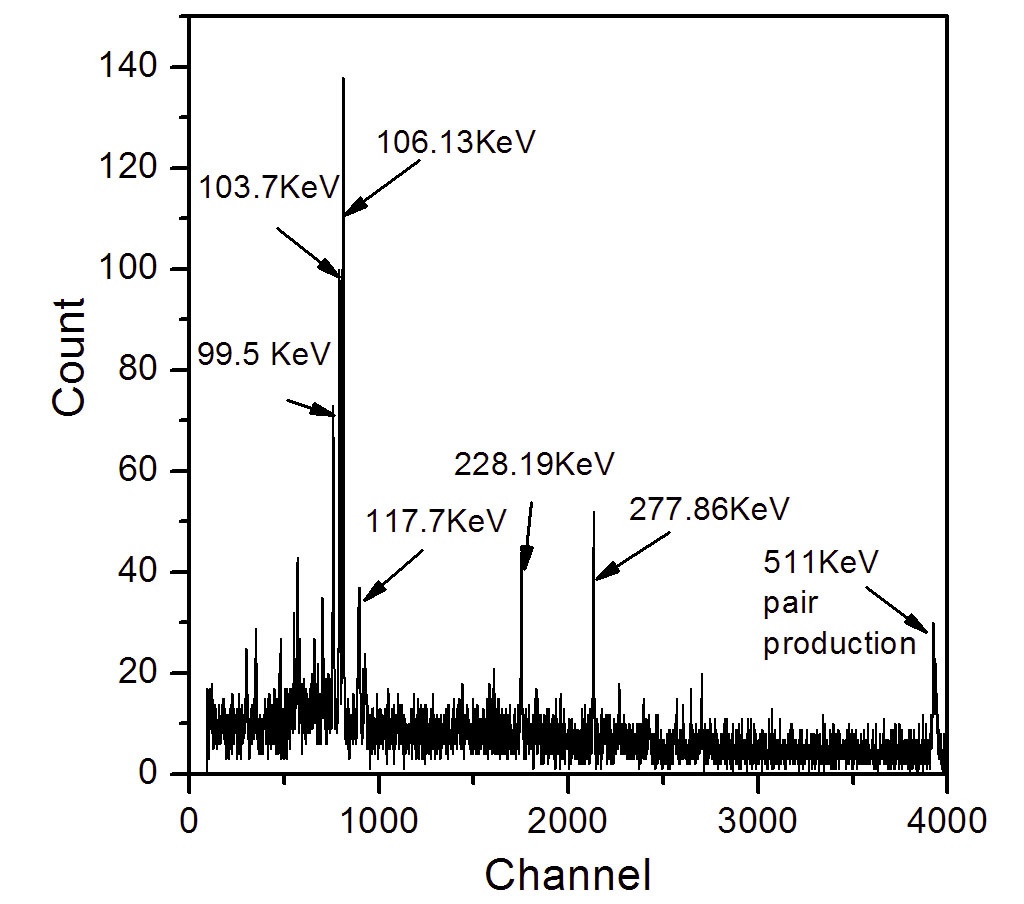
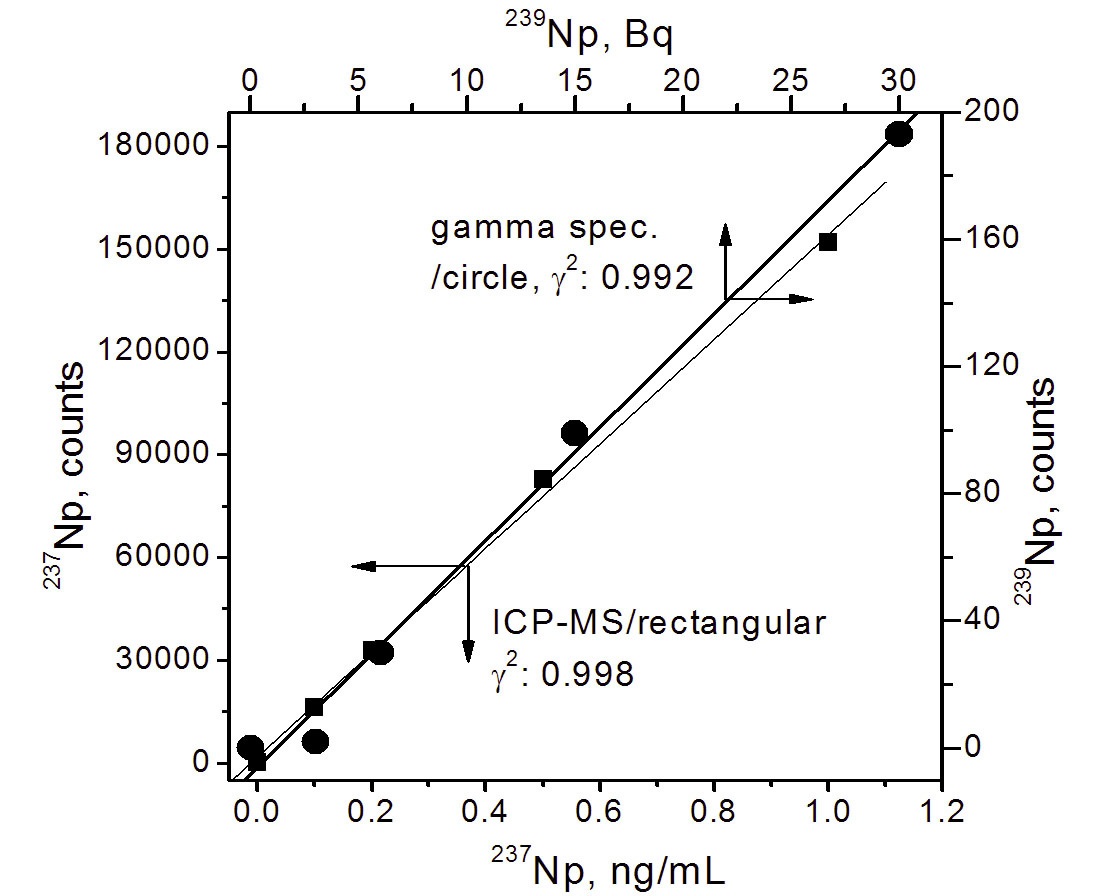
First, two calibration curves for 239Np and 237Np were obtained through gamma spectrometry and ICP-MS spectrometry, respectively. The gamma activities of 239Np were measured at 277.86 KeV for 3, 6, 15, and 30 Bq for 5000 sec. The gamma spectrum of 239Np has a number of peaks at energies of around 90 to 300 KeV, as shown in Fig. 2. In this study, the peak at 277.86 KeV was selected for measurements because this peak has the highest energy and a relatively high branching ratio (14.1%). The peaks at about 100 KeV with high peak intensities were not taken owing to a high background effect. The calibration curve showed a good linearity (
MS, the calibration curve also expressed a good linearity (
the measurements by ICP-MS and gamma spectrometry, respectively. The gamma activity of 239Np was measured within as short a time as possible (<5-8h) after separation from 243Am owing to its short half life (t1/2=2.35d). Thus, decay corrections were made for the time interval between the end of the separation and the start of the measurement, and during the measurement time using equations (2) and (3), respectively. Finally, the measured activity (Bq) of 239Np was converted into weight (ng) using a specific activity of 239Np (1.27x10-7 ng/Bq).
where
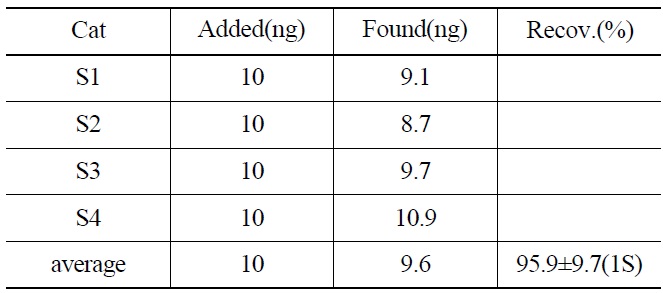
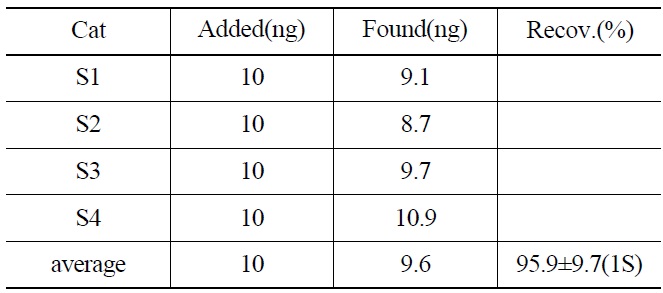
Recovery Yield of 237Np from the Synthetic Samples by an Isotope Dilution Mass and Gamma Spectrometry
3.2 Determination of 237Np in the Spent Fuel Samples
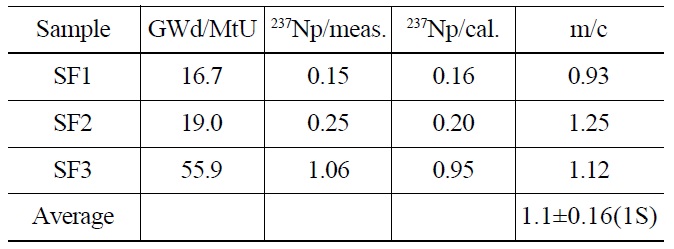
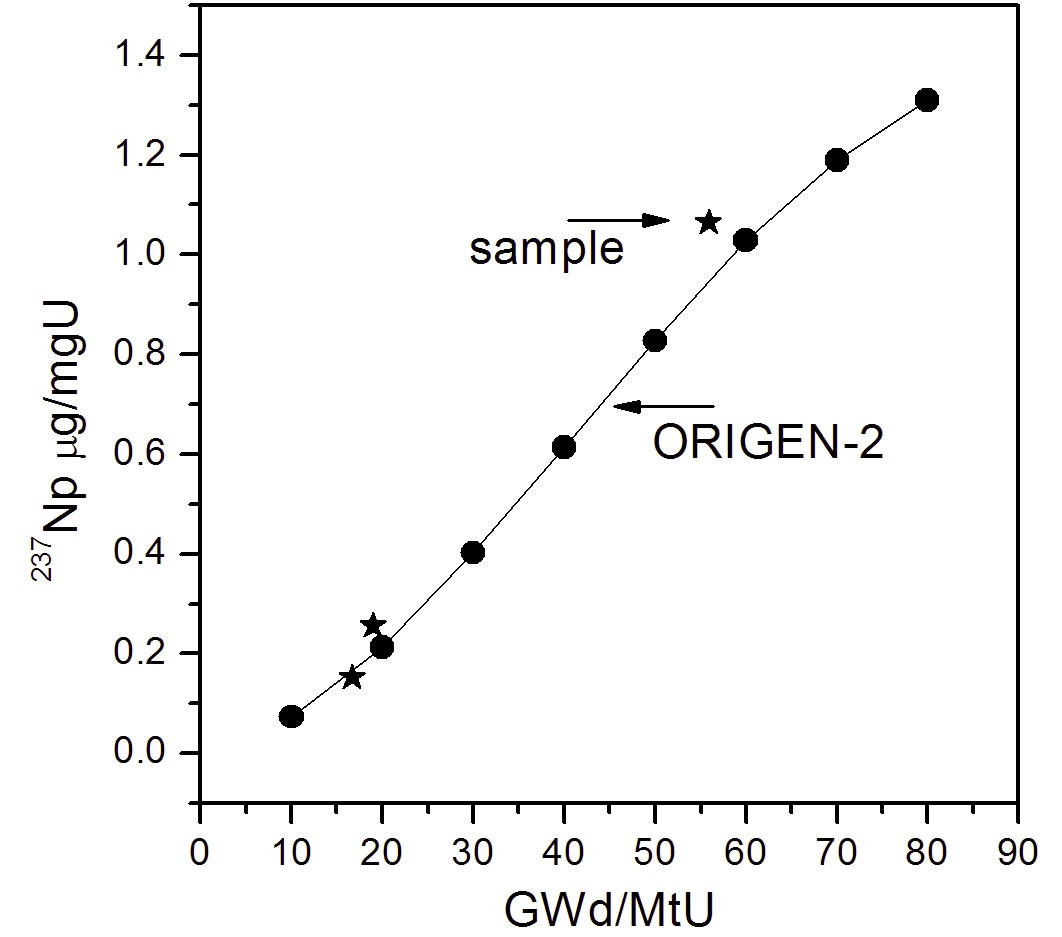
The contents of 237Np in the spent fuel samples were determined using an isotope dilution method, as mentioned above. The measured values of 237Np were 0.15, 0.25, and 1.06 μg/mgU for SF1, SF2 and SF3, respectively (Table 3) and were compared with those predicted by a calculation using the ORIGEN-2 code. The measurement values (m) agreed with the calculation values (c) as a ratio (m/c) of 0.93, 1.12, and 1.25, respectively, within about 10% difference on average. Fig. 4 shows a correlation curve between the measurement and the calculation as a function of burnup. In the literature reporting 237Np content in spent nuclear fuels, the measurement values of 237Np obtained from the PWR spent fuel from the Takahama-3 reactor were found to be in good agreement to within 4% difference at a high burnup (30-47.25 GWd/MtU) and showed a range of 34.2-71.8% of differences at a low burnup (7- 28.9 GWd/MtU) compared to the calculated values [16]. The contents of 237Np in the MOX fuel irradiated up to 120 GWd/MtU in the Mark-II reactor of JOYO in Japan were greatly biased from the calculated values [17]. From these results a higher deviation of the measurement from the calculation was observed in low burnup and MOX fuel. This means
[Table 3.] The Contents of 237Np in Spent Fuel Samples (unit: mg/mgU)
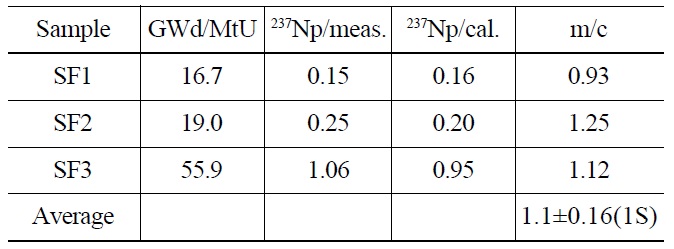
The Contents of 237Np in Spent Fuel Samples (unit: mg/mgU)
that the deviation increases as the content of 237Np goes down and the fuel type is more complicated. In this work, the measurement values showed a fairly good agreement (~10% difference on average) with the calculated values compared to other works. However, it is difficult to find a correlation between the deviation and the fuel burnup owing to a lack of data.
In this work, ICP-MS was taken for the determination of 237Np in spent nuclear fuel samples instead of alpha spectrometry in the previously developed isotope dilution method. Two calibration curves for 237Np and 239Np were established by using ICP-MS and gamma spectrometry, respectively, for the verification of the measurement values. Finally, this method was applied to the three PWR spent nuclear fuel samples after a recovery yield test from the synthetic samples. The result showed that the measurement values agreed with the calculated values within an acceptable error range.
In the future, this method will be validated using the 237Np data obtained through other methods and will also be used further to contribute to the buildup of 237Np database in the spent nuclear fuels.