Silk is a naturally occurring material from silkworms and consists of fibroin and sericin. Silk has been used as a high quality textile material for a long time. Sericin is usually removed from silk through a degumming process that significantly improves the luster and touch feeling of silk fiber. Considering that silk contains about 25% sericin, a high amount of sericin is lost through the degumming process because most people believe that sericin is not a useful material. However, recent studies have focused on determining the useful and valuable properties of sericin, and the results of these studies have established many unique and valuable properties of sericin as a biomaterial.
Tsubouchi
In addition to these applied studies, many studies into the extraction of sericin from silk have also been performed to obtain sericin with a higher molecular weight (MW) or better functional properties. It is generally accepted that molecular degradation of sericin occurs during hot water extraction and that degradation of sericin during extraction depends on the extraction method. To date, the most effective extraction method for obtaining high- MW sericin was to extract sericin using 8 M urea solution with 5% 2-mercaptoethanol (Takasu
The molecular conformation of silk protein has been studied using Fourier-transform infrared (FTIR) spectroscopy because it plays an important role in determining the properties of silk protein. Kim
In this study, we extracted sericin using various extraction solutions and times and prepared its film. We examined the effect of the extraction method on the molecular conformation of the sericin film. Additionally, we examined the effect of the film side (air-facing or petri-dish plate-facing) on the molecular conformation of the sericin film using an FTIR spectrometer with attenuated total reflection (ATR) geometry.
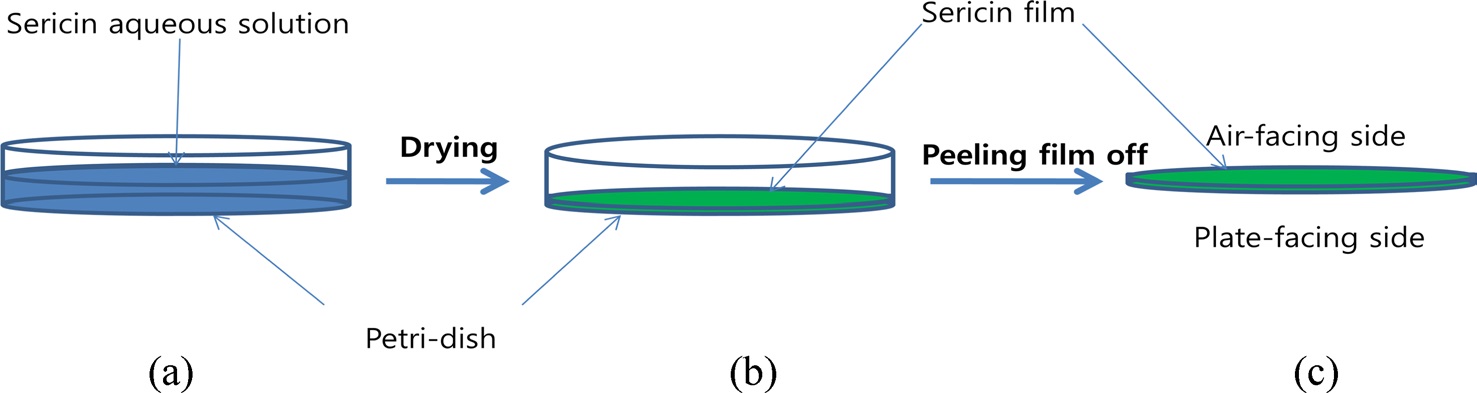
Baekokjam silkworm cocoons were used to extract silk sericin. Silk sericin was extracted using 3 different extraction solutions: distilled water, citric acid solution, and urea solution. For the water extraction, sericin was extracted by immersing the cocoons in water at 100℃ for 30~120 min. For the citric acid extraction method, the cocoons were treated in a 1.25% (w/v) aqueous citric acid solution at 100℃ for 30 min. The extracted sericin solution was dialyzed in distilled water using a dialysis tube (MW cutoff = 12,000~14,000) for 7 days to remove the citric acid. For the urea extraction, the cocoons were treated in an 8 M aqueous urea solution at 85℃ for 30 min after immersion in the same solution at room temperature for 30 min. The extracted sericin solution was dialyzed to eliminate urea using the same method as that used in citric acid extraction. All extracted sericin solutions were filtered into non-woven fabric twice before dialysis (for acid and urea extraction) or drying (hot water extraction). Aqueous sericin solutions from the 3 different extraction methods were poured into petri-dishes and dried at 40℃ in a drying oven to form the sericin film.
>
FTIR measurements and determination of the crystallinity index
The FTIR (Nicolet 380; Thermo Fisher Scientific, USA) spectra of the sericin films were obtained using the ATR method. To examine the effect of the sericin film side, 2 different sides (air-facing and plate-facing) of the sericin film (Fig. 1) were measured using ATR geometry. The crystallinity index was
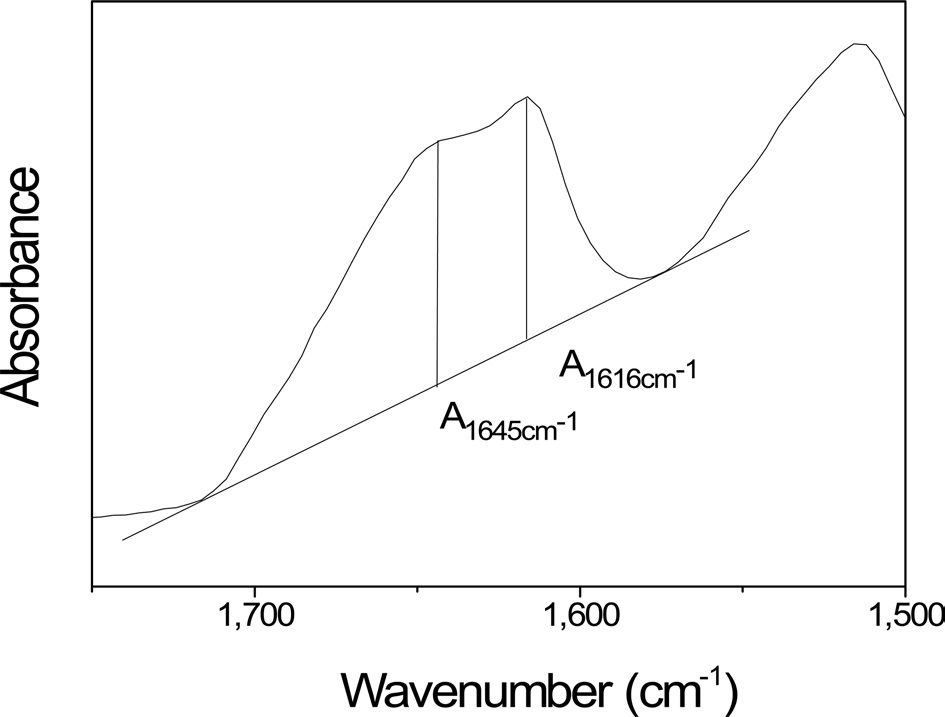
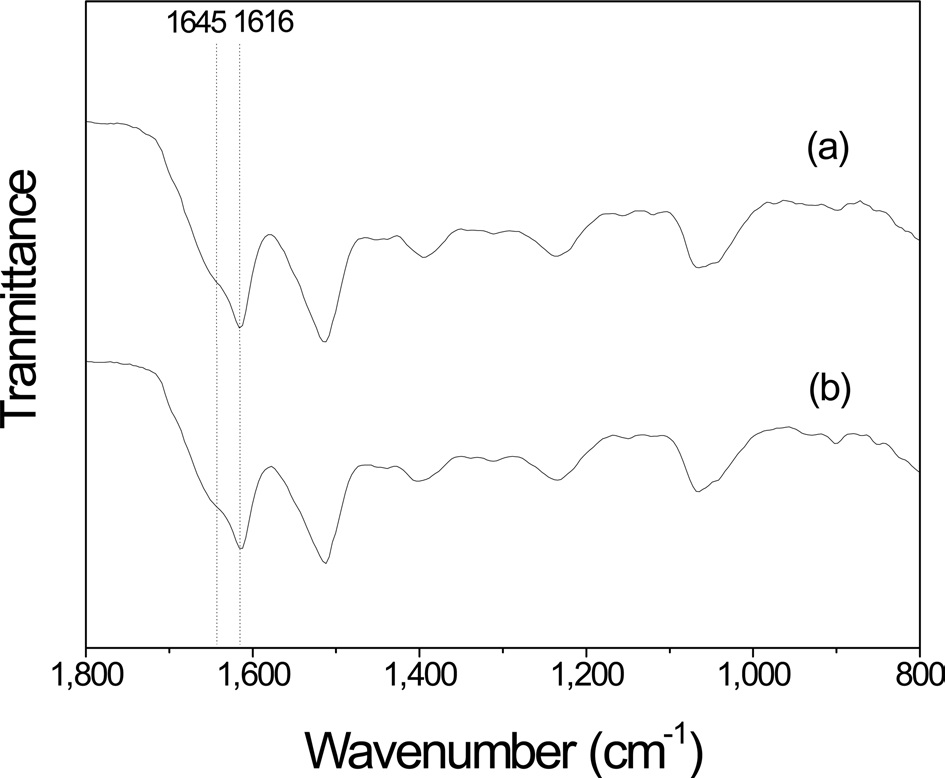
calculated using an absorbance of 1616 and 1645 cm-1 from the FTIR spectrum, which corresponded to the β-sheet and random coil conformations, respectively (Fig. 2). The crystallinity index was calculated using the following equation:
A1616 cm-1, Absorbance at 1616 cm-1
A1645 cm-1, Absorbance at 1645 cm-1
To obtain the average and variations of the crystallinity index, FTIR measurements were performed on 7 different parts of the sericin film.
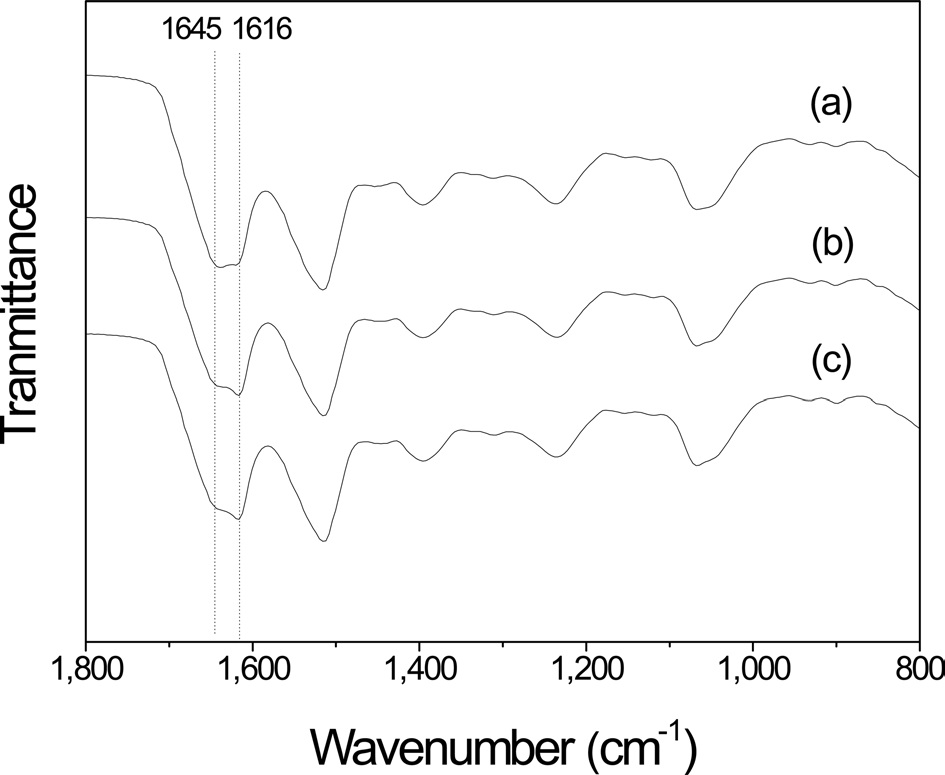
The FTIR spectra of the air-facing side of the sericin film prepared using hot water extraction with different extraction
times are shown in Fig. 3. After extraction for 30 min, 2 overlapping peaks at 1645 and 1616 cm-1 in amide I band, which corresponded to a random coil and β-sheet conformation (Um
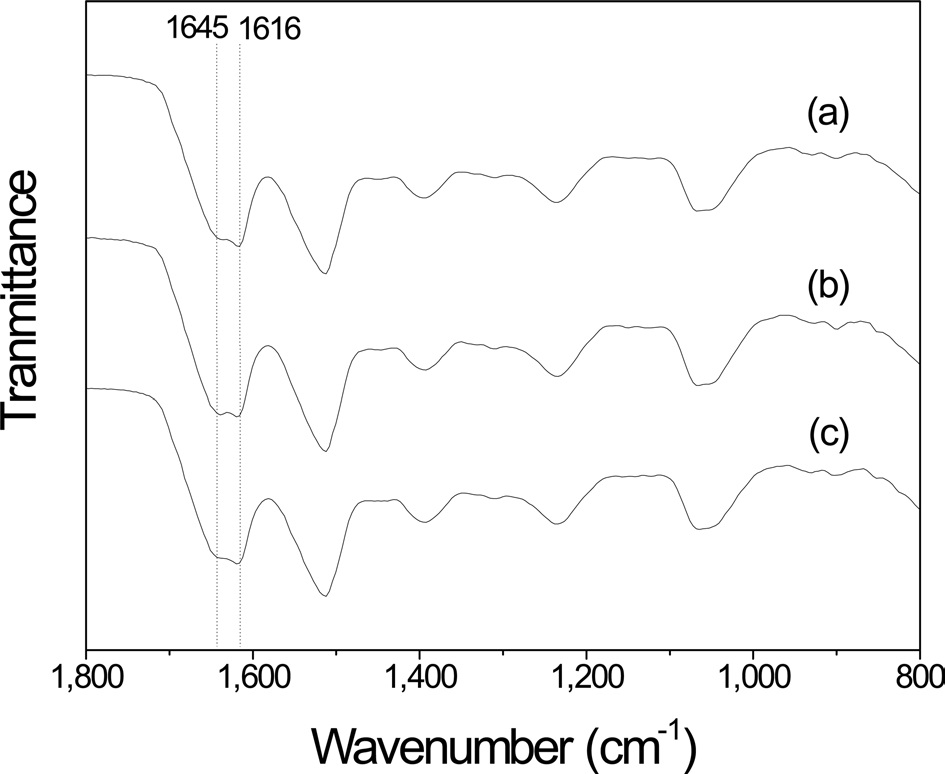
The FTIR results of the plate-facing side (petri-dish facing side) of sericin film were different from those of the airfacing side of film. After extraction for 30 min (Fig. 4), two IR absorption peaks were observed at 1645 and 1616 cm-1; the latter peak slightly stronger than the former. However, unlike the FITR spectra of the air-facing side of the sericin film, that of the platefacing side of the sericin film did not change with a change in the extraction time.
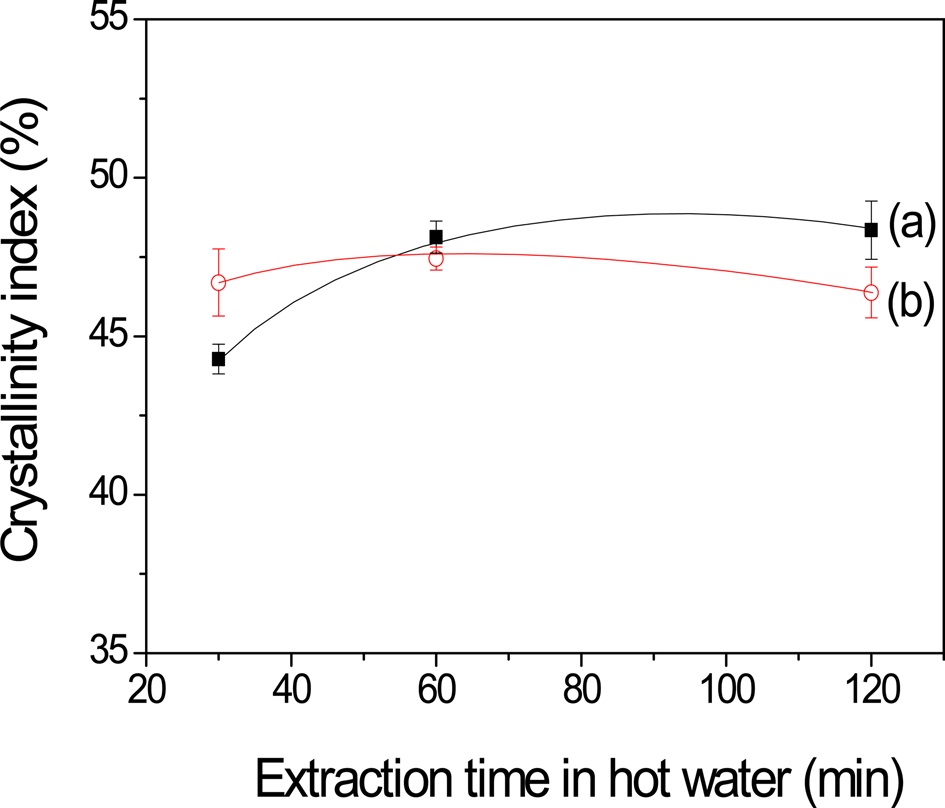
The crystallinity index calculated from amide I band (Fig. 5) clearly indicates different trend of crystallinity of the sericin film between the air-facing and the plate-facing sides. The crystallinity index of the air-facing side of the silk sericin film prepared by extraction for 30 min was 44.3%. The crystallinity index increased up to 48.3% when the extraction time increased to 120 min. In contrast, for the plate-facing side of the sericin film, the crystallinity index ranged from 46 to 48%, and it did not almost change with a change in the extraction time.
The FTIR spectra of the air-facing side of the sericin films prepared by extracting sericin in an aqueous citric acid solution and an aqueous urea solution are shown in Fig. 6. The two sericin films showed a strong IR absorption peak at 1616 cm-1 with a weak shoulder peak at 1645 cm-1. This indicates that these films contained a high amount of β-sheet crystallite. The FTIR spectra of the plate-facing side of the sericin films extracted using the two solutions is shown in Fig. 7. The FTIR spectra of these films were similar to those of the air-facing side of the
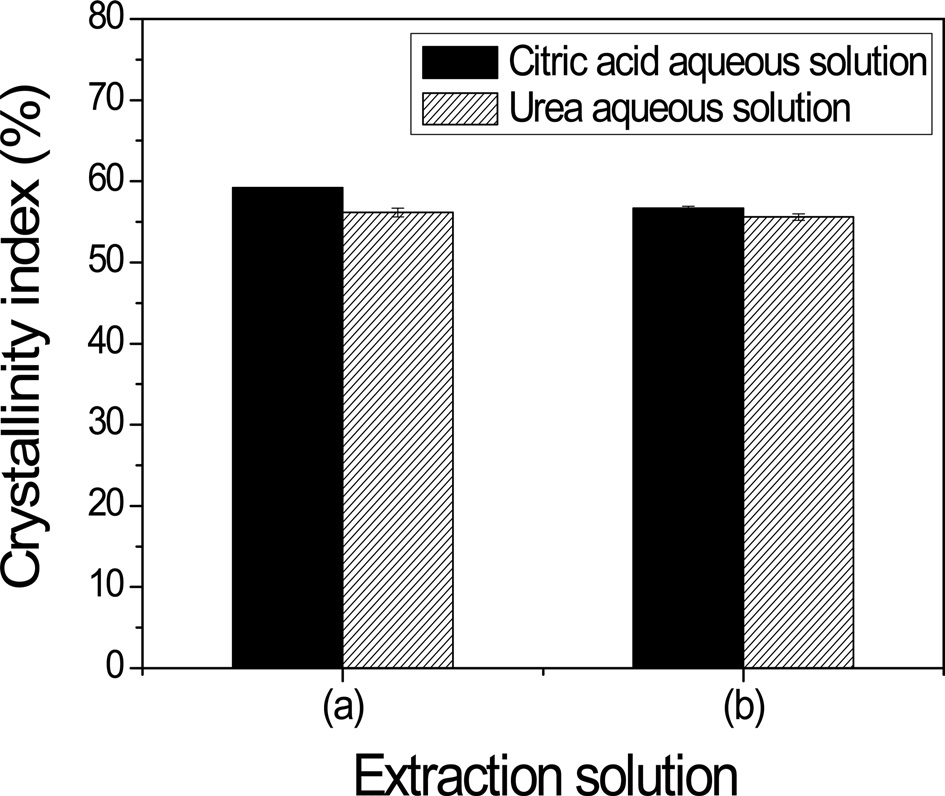
sericin films. However, the trend of crystallinity index of the airfacing side of the film was different from that of the plate-facing side of the film (Fig. 8). The crystallinity index of air-facing side of the sericin film extracted from an aqueous citric acid solution was higher than that of the film obtained from an aqueous urea solution by 2.5% (Fig. 8(a)). However, the crystallinity indices of the plate-facing side of two sericin films extracted using different extraction solutions were not significantly different.
The results of the FTIR studies on the sericin films for the different film side (air- or plate-facing) following different extraction conditions (extraction time in hot water or extraction solvent) showed several interesting features.
Firstly, the film side (air-facing or plate-facing) showed different trends of molecular conformation and crystallinity indices of the silk sericin films. In the case of air-facing surface of the film, the molecular conformation of sericin was significantly influenced by (1) the extraction time in hot water and (2) the type of extraction solution. However, no remarkable differences were observed for the plate-facing surface of the sericin film in terms of the above two factors. Although the exact mechanism underlying this behavior cannot be explained in this study, our findings indicate that different crystallizations of sericin take place on the air-facing surface and on the bulk side (or inside) of the sericin solution.
A second interesting feature is the increase of the crystallinity index for the air-facing sericin film surface following increased extraction time. It seems that this is related to the MW of sericin. That is, the hydrolytic molecular degradation of sericin takes place during the extraction of sericin in hot water. Therefore, the MW of sericin decreases with an increase in the extraction time of sericin in hot water. This indicates that the crystallinity of sericin increases when the MW of sericin decreases. This may be because of the difference of molecular mobility between sericins of different MWs. That is, as the MW of sericin decreases, the molecular mobility increases. The sericin molecules can arrange and crystallize more easily when their mobility is increased. In contrast, the lower molecular mobility of the higher MW of sericin might restrict the molecular arrangement of sericin resulting in a lower level of β-sheet crystallite formation.
Lastly, the crystallinity indices of the sericins extracted with the aqueous citric acid solution and aqueous urea solutions were much higher than those for the sericin films obtained through hot water extraction. It is thought that this result is intimately related to the different preparation methods of the sericin film and the gelation behavior of sericin. That is, the sericins extracted from hot water were prepared by casting aqueous sericin solutions in petri-dishes just after the hot water-extracted sericins were added. In contrast, the sericin films extracted with the aqueous citric acid and urea solutions were dialyzed to remove the citric acid and urea respectively. Thus, after 7 days dialysis, aqueous sericin solutions are obtained and the films are prepared by drying the solutions. However, after dialysis, the sericin solutions contain many gels because sericin gels easily and some sericin became gel during the dialysis. When the gelation of sericin takes place, it is well known that β-sheet crystallization occurs. Therefore, a stronger IR absorption peak and higher crystallinity index were observed for the sericin films extracted with the aqueous citric acid and urea solutions.
It is also interesting to note that the sericin film extracted with the aqueous citric acid solution on the air-facing surface had a higher crystallinity index than when extracted with the urea solution, as can be seen in Fig. 8. This might be due to the different gelation behaviors of sericin between the two extraction methods. The aqueous citric acid solution is acidic because citric acid is an acid. After the extraction of sericin with the aqueous citric acid solution, the pH of the sericin citric acid solution was lower than the isoelectric point of sericin (i.e. pH 4.0 (Voegeli