기존 탄화수소계 세정제는 낮은 인화점으로 인하여 화재, 폭발과 같은 문제점을 가지고 있다. 인화점을 높이기 위해 불소가 포함된 난연성 세정제 7종을 개발하였다. 새롭게 합성한 세정제를 한국석유관리원에서 국가산업표준 방법으로 분석하여 물성을 측정하고 세정성 평가를 수행하였다. 물성 측정 결과, 인화점이 탄화수소계 세정제보다 높은 것을 확인하였다. 세정력 평가를 위하여 가로 60 mm, 세로 40 mm 크기의 스테인리스강 기판에 인발유, 아비에트산, 절삭유, 윤활유를 각각 도포하여 합성한 세정제(1~7)에 넣어 침적한 후 세정력을 평가하였다. 최대 5분간 침적하였을 때 인발유는 80~96%, 아비에트산은 82~97%, 윤활유는 86~93% 정도의 높은 세정력을 보였다. 그러나 절삭유에서는 60~87% 정도로 다른 오일보다 낮은 세정력을 보였다. 따라서 약간의 차이는 있지만 대체적으로 우수한 세정력을 보여 산업세정분야에 적용이 가능함을 나타내었다.
A chlorine-based solvent such as 1,1,2-trichloroethylene (TCE), 1,2-dichloropropane, and methylene chloride has been widely used for its excellent cleaning ability. However, since chlorinebased solvent has high volatility, it is harmful to both human body and environment[1-3]. The hydrocarbon-based cleaning agent has been used as the substitute, but the problems that related to flammability and drying are being blamed. Flammability is important because many industrial facilities are not equipped with safely handle flammable hydrocarbons. In addition, hydrocarbon containing an aromatic hydrocarbon, such as benzene, toluene, and xylene are being regulated due to its high toxicity on human body and environmental pollution[4,5]. Therefore, development of new environmentally acceptable cleaning agent would be highly desirable.
Fluorinated organic compound in which hydrogen atoms has been replaced by fluorine atoms has a higher flash point[5] than that of the fluorine free compound. It is also known that ester compounds have an important role in cleaning dirty metal surfaces[7,8].
Herein, we describe a synthesis and evaluation of fluorinated ester compounds which exhibit low toxicity to human body due to their low flammability.
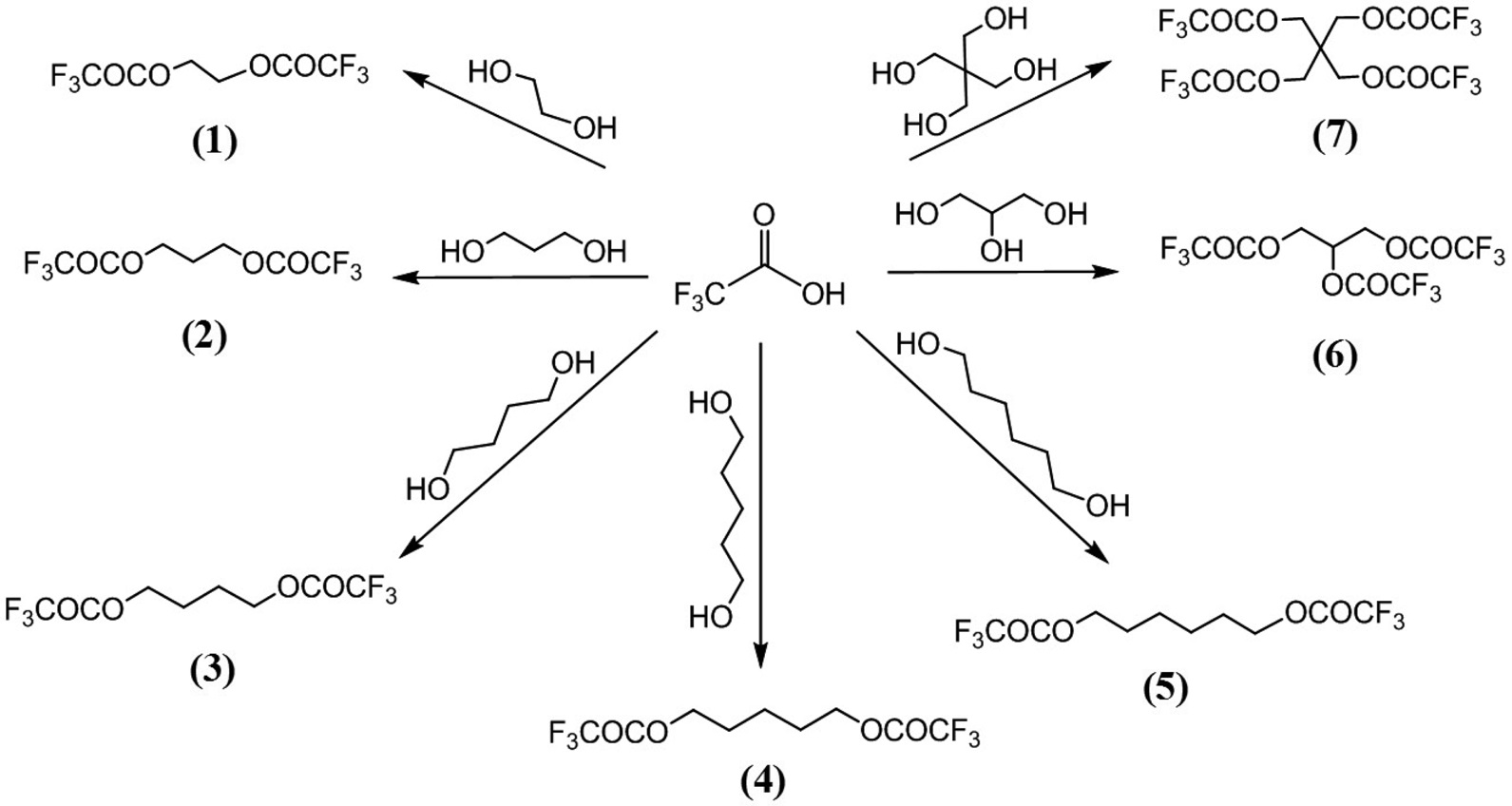
The core structure of the new fluorinated target compounds (1-7) is ester group which connected to polyol chain. The synthesis of target compounds is outlined in Scheme 1. The ester compounds (1-7) were synthesized by esterification reaction of trifluoroacetic acid (TFA) with polyols in the presence of acid catalysis in moderate yield.
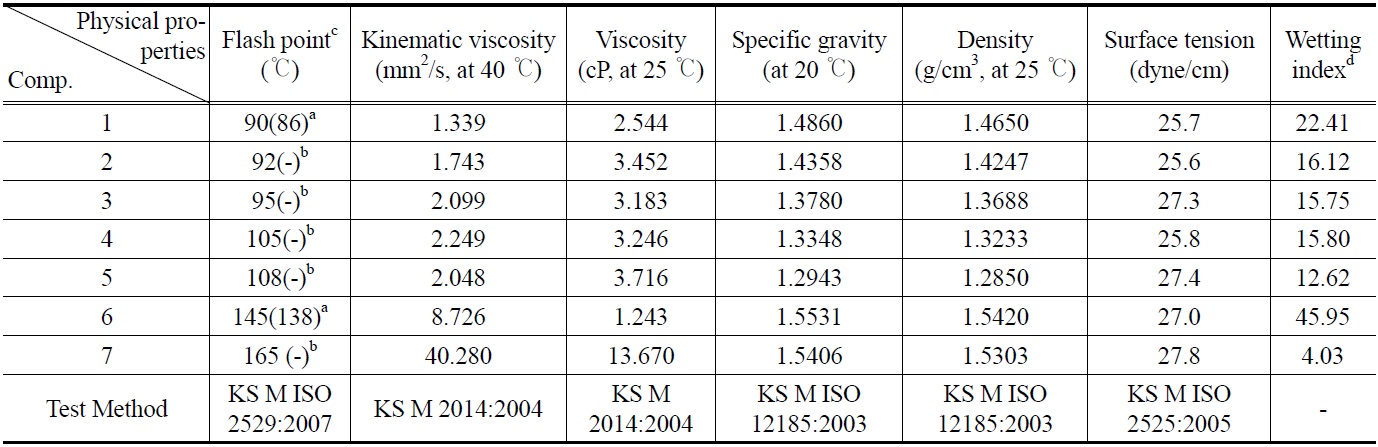
All synthesized ester compounds (1-7) were estimated for their possibility of cleaning agent by examining physical properties which were conducted by Korea Institute of Petroleum Management by using standard methods. All compounds have various
[Table 1.] Physical properties of compounds (1-7)
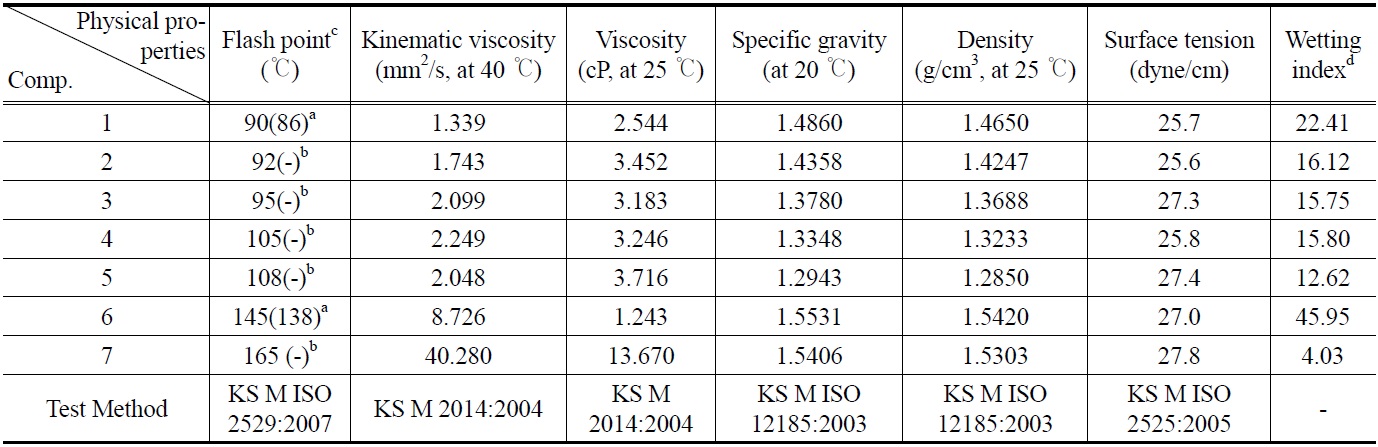
Physical properties of compounds (1-7)
good physical properties which are almost similar to other cleaners[9,10]. When flash point of all compounds (1-7) was compared with that of non-fluorinated (hydrogen substituted) compounds, the data indicated all compounds show high flash point that related to flammability (Table 1). This finding suggests that all synthesized compounds (1-7) can be a new candidate as nonflammable cleaning agent.
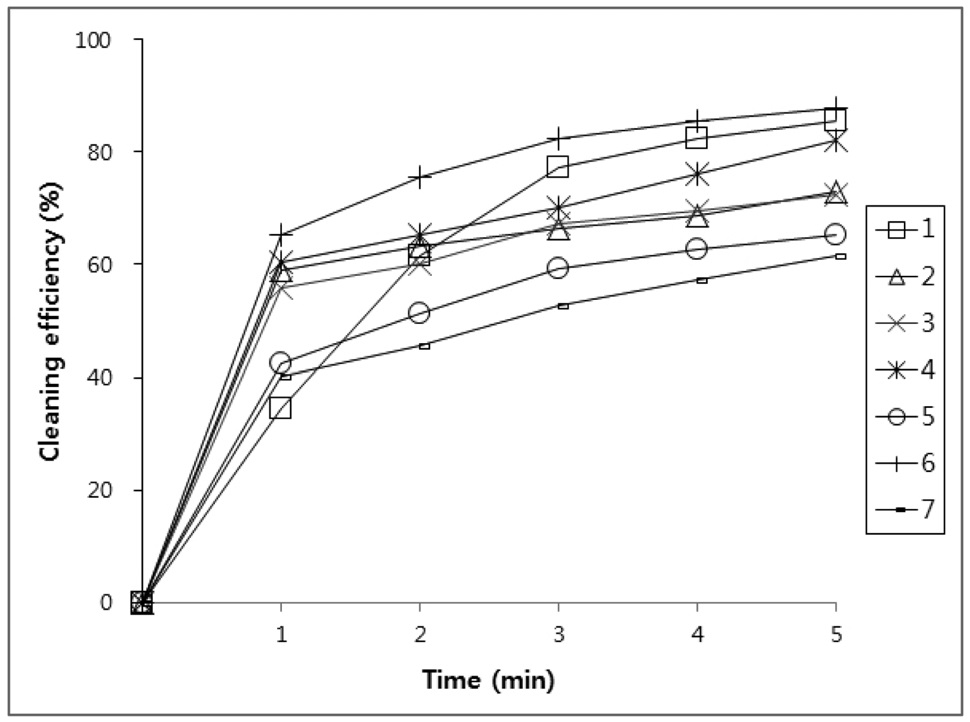
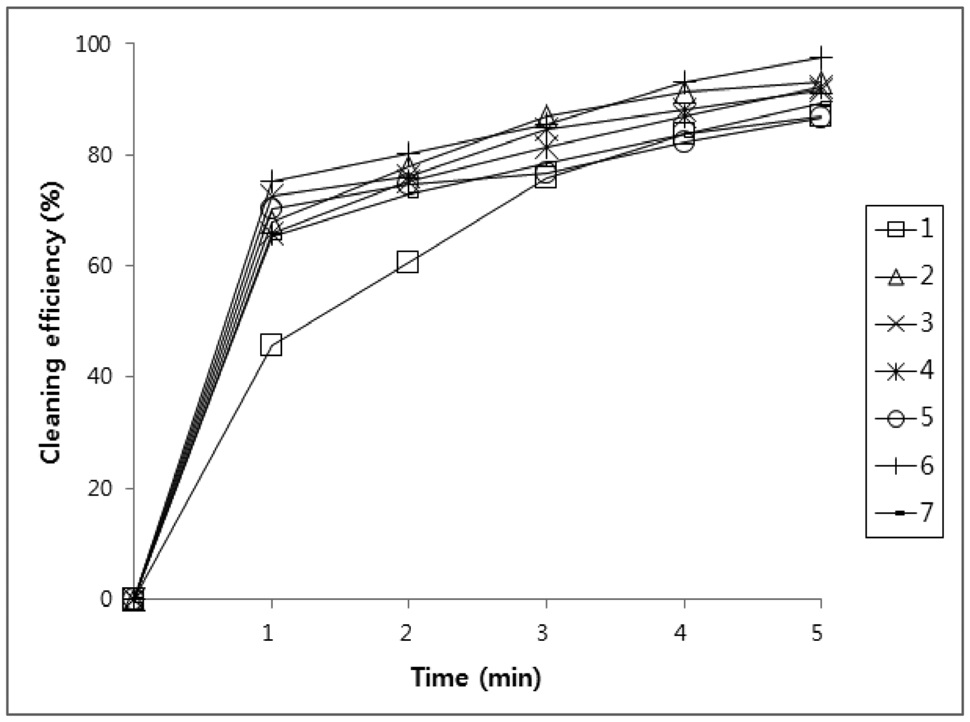
All new compounds (1-7) were evaluated for their cleaning efficiency by simple immersion cleaning method. A specimen was prepared by cutting in 60 mm × 40 mm size of stainless steel plate and weighted. The surface of the above specimens was applied with four kinds of contaminants, paraffin-based drawing oil, flux abietic acid, water-insoluble cutting oil, and lubricating oil which were used for various industrial processing.
The weight that was added with the specimen was recorded down to four places of decimals. The above specimens were immersed in 100 mL of compounds (1-7) for 1 to 5 minutes to dissolve oil in the cleaning agent. After that, the specimens were dried and weights were measured.
Three tests were performed on each compound. Using the above
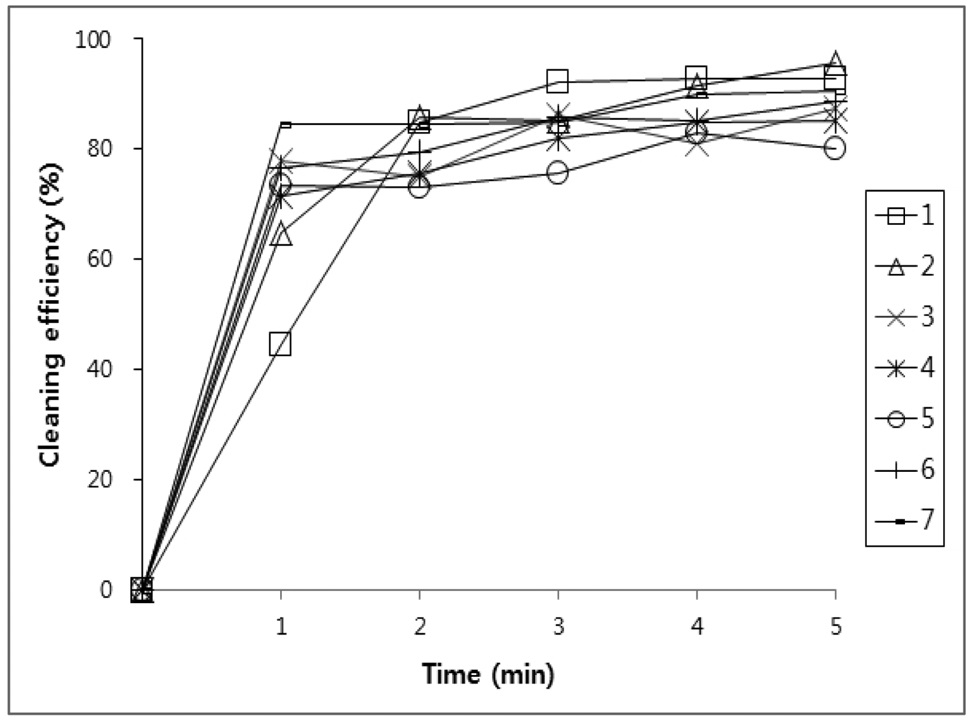
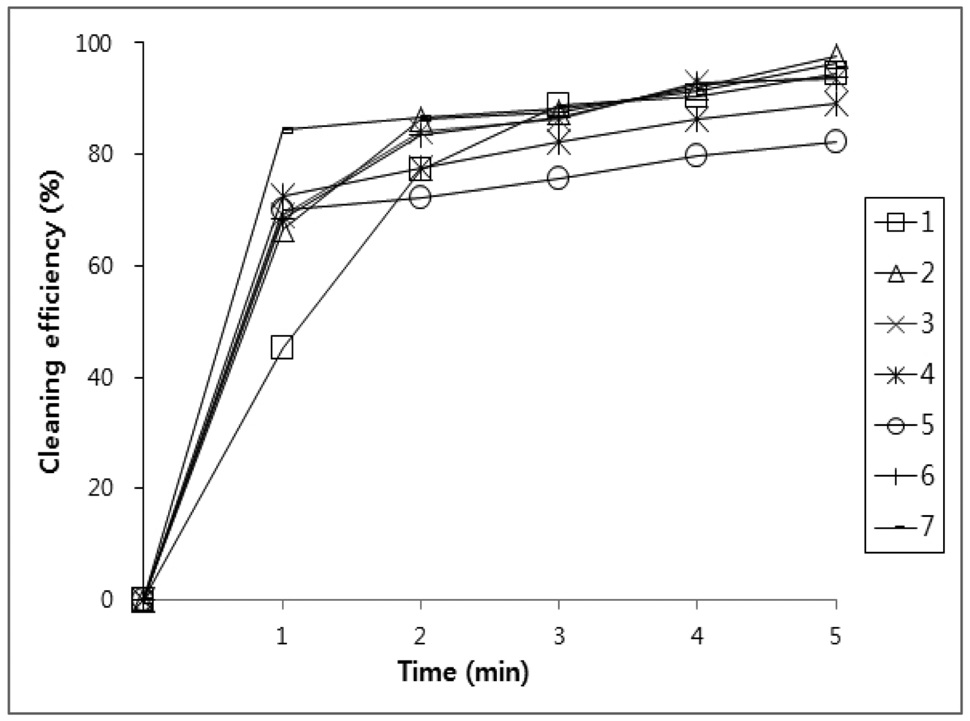
values, the amount of oil that was initially adhered on the specimen and the amount oil that was removed by the cleaning agents were calculated and putted on a percentage basis in Figure 1, 2, 3, and 4.
Figure 1, 2, 3 and 4 indicate that all compounds (1-7) show some low cleaning efficiency for four contaminants in 1-2 minutes dipping. However, gradual increment of dipping time can raise cleaning efficiency. Although the data in Figure 3 indicate that all compounds exhibit lower cleaning ability toward cutting oil, it is observed that in the case of the present study more than 80% of pollutants on the surface were almost removed within 5 minutes. Accordingly, one might suggest that these compounds (1-7) are suitable for new nonflammable cleaning agents.
3.2. Synthetic methods of cleaning agents
Generally, all proton nuclear magnetic resonance (NMR) spectra were recorded on a Varian Unity Inova spectrometer at
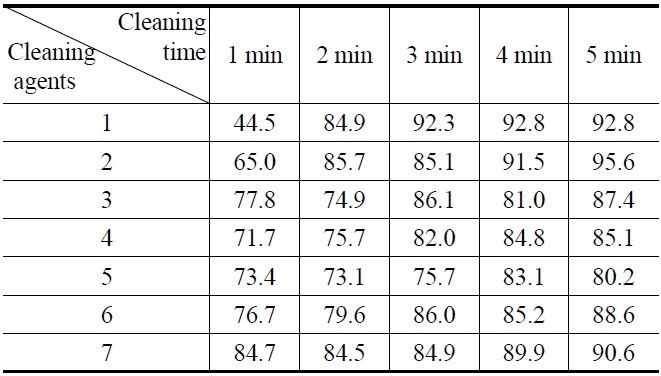
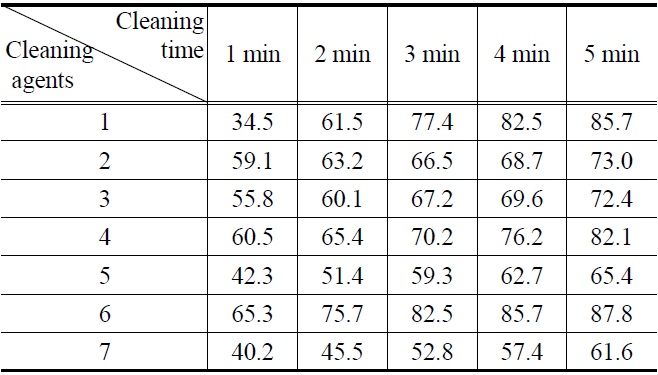
[Table 2.] Cleaning efficiency (%) of drawing oil by (1-7) cleaning agents
Cleaning efficiency (%) of drawing oil by (1-7) cleaning agents
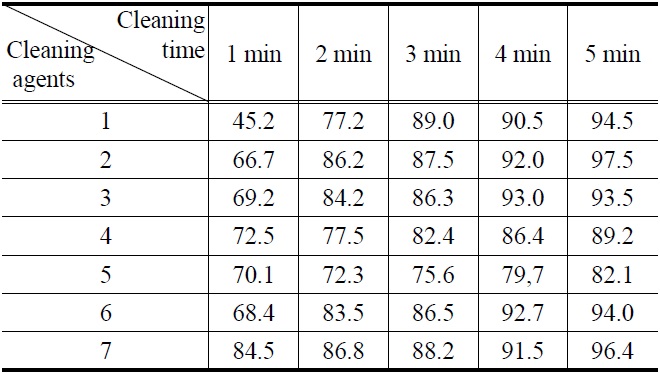
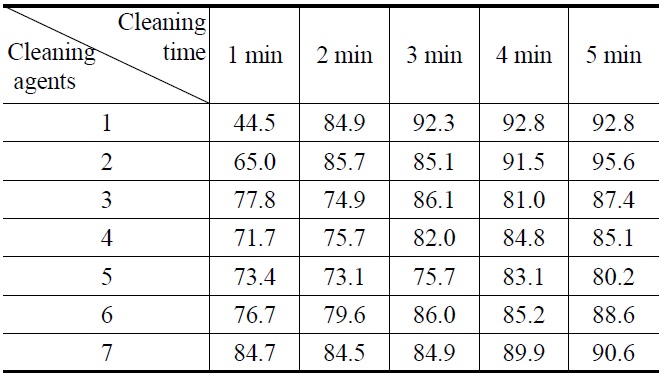
[Table 3.] Cleaning efficiency (%) of abietic acid by (1-7) cleaning agents
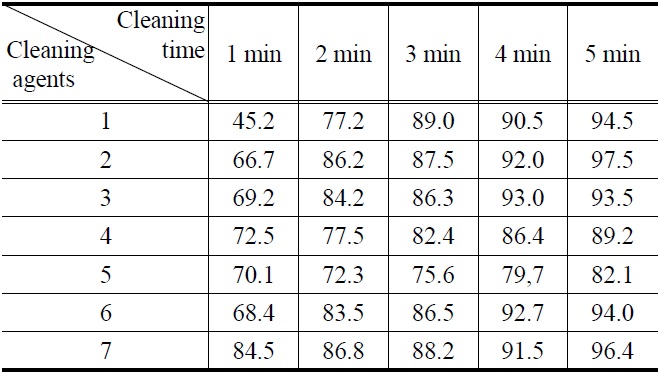
Cleaning efficiency (%) of abietic acid by (1-7) cleaning agents
[Table 4.] Cleaning efficiency of cutting oil by (1-7) cleaning agents
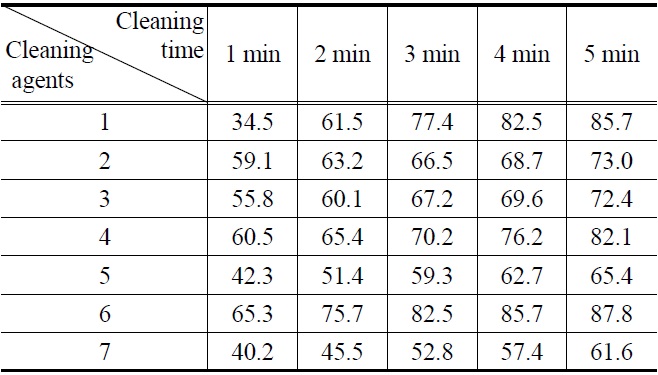
Cleaning efficiency of cutting oil by (1-7) cleaning agents
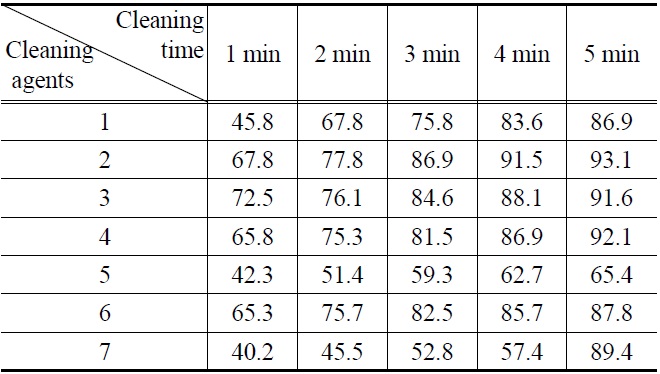
[Table 5.] Cleaning efficiency (%) of lubricating oil by (1-7) cleaning agents
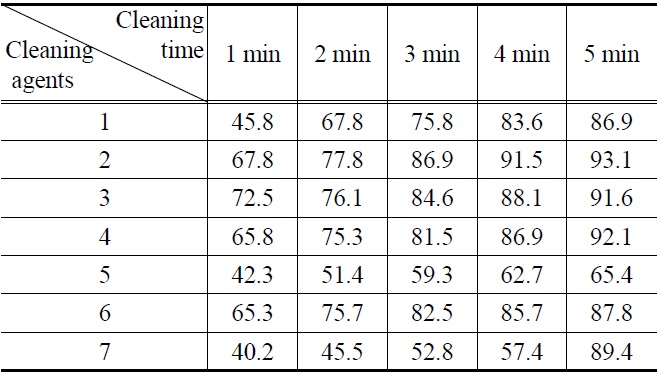
Cleaning efficiency (%) of lubricating oil by (1-7) cleaning agents
300 MHz and are reported in parts per mllion (ppm) on the δ scale relative to chloroform-
Ethane-1,2-diyl bis(2,2,2-trifluoroacetate)(1)
To a stirred solution of ethylene glycol (2.0 kg, 28.95 mol) and p-TsOH (396 g, 2.89 mol) in toluene (3.0 L) was added slowly TFA (6.65 L, 86.85 mol) at room temperature. The resulting mixture was heated to remove water for 3 hours. After cooling, methylene chloride (4.0 L) was added to the reaction mixture. The organic layer was washed with water (2 × 4.0 L) and dried with MgSO4, filtered, and solvent evaporated under reduced pressure. The crude product was purified by vacuum distillation (20 mmHg, 100-105 ℃) to afford 1 (4.98 kg, 81%); 1H NMR (300 MHz, CDCl3) δ 4.68 (s, 4H).
Propane-1,3-diyl bis(2,2,2-trifluoroacetate) (2)
(20 mmHg, 110-115 ℃, 80%), 1H NMR (300 MHz, CDCl3) δ 2.2 (m, 2H), 4.5 (t, J = 7.0 Hz, 4H).
Butane-1,4-diyl bis(2,2,2-trifluoroacetate) (3)
(20 mmHg, 115-120 ℃, 77%), 1H NMR (300 MHz, CDCl3) δ 1.90 (t, J = 6.8 Hz, 4H), 4.4 (t, J = 6.8 Hz, 4H).
Pentane-1,5-diyl bis(2,2,2-trifluoroacetate) (4)
(20 mmHg, 125-128 ℃, 73%), 1H NMR (300 MHz, CDCl3) δ 1.50 (m, 2H), 1.9 (m, 4H), 4.4 (t, J = 6.5 Hz, 4H).
Hexane-1,6-diyl bis(2,2,2-trifluoroacetate) (5)
(20 mmHg, 127-130 ℃, 69%), 1H NMR (300 MHz, CDCl3) δ 1.45 (m, 4H), 1.8 (m, 4H), 1.85 (m, 4H), 4.4 (t, J = 6.8 Hz, 4H).
Propane-1,2,3-triyl tris(2,2,2-trifluoroacetate) (6)
(10 mmHg, 139-142 ℃, 70%), 1H NMR (300 MHz, CDCl3) δ 4.20-5.05 (m, 5H).
2,2-Bis((2,2,2-trifluoroacetoxy)methyl)propane-1,3-d iyl bis(2,2,2-trifluoroacetate) (7)
(10 mmHg, 150-154 ℃, 60%), 1H NMR (300 MHz, CDCl3) δ 4.40 (s, 8H).
In summary, we have prepared seven new unprecedented fluorinated ester compounds (1-7) in moderate yield from TFA and corresponding polyols. These compounds can be applicable to various industrial cleaning fields as nonflammable cleaning agent because of their good physical properties and cleaning abilities for contaminants. The future work including formulation of (1-7) with other solvents to find out nonflammable cleaning systems is under investigation in our laboratory.