Herbs are considered less toxic than synthetic drugs, and considerable research efforts are being made to develop new drugs from natural products.12 However, following an increase in the consumption of herbal medicines, potential risks associated with pharmacokinetic interactions have been reported. Herb extracts and components in herb extracts are known to either induce or inhibit drug-metabolizing enzymes elsewhere; St John’s wort and ginko biloba provide two better known examples.3-5 Therefore, possible herb-drug interactions, particularly through the inhibitory effects of pharmacological components on CYP enzymes, should be considered during the drug development process.
Mollugin (isolated from
In a previous study, we found that mollugin might cause herb-drug interactions via the selective inhibition of CYP1A2 in pooled human liver microsomes (HLMs).14 Although several studies have been conducted on the pharmacological effects of mollugin, its effects on CYP enzymes
The mollugin used in present study was isolated from
>
Animals treatments and preparation S-9 fractions
Specific pathogen-free male ICR mice (28 to 33 g) were obtained from the Orient Co. (Seoul). The animals were purchased at 4 weeks of age and then acclimated for at least 2 weeks. Upon arrival, animals were randomized and housed three per cage in temperature and humidity controlled (23 ± 3 ℃ and 50 ± 10% RH) animal quarters under a 12 h light/12 h dark cycle at an intensity of 150-300 Lux. All animal procedures complied with the guidelines issued by the Society of Toxicology (USA; 1989).
To investigate the CYP modulating effects of mollugin, male ICR mice were orally administrated 20, 40, or 80 mg/kg of mollugin in solution (Ethanol:PEG 400:Water = 5:40: 55) once daily for 3 days. Animals were necropsied at 24 h after last treatment. Following blood collection, livers were homogenized with four volumes of ice-cold 0.1 M potassium phosphate buffer (pH 7.4), and centrifuged at 9,000 × g for 20 min at 4 ℃. Aliquots of the supernatant (S-9 fractions) were store at ?80 ℃ until required. Protein contents in S-9 fractions were determined using bovine serum albumin as a standard.15
S-9 fractions were prepared from livers to determine CYP-associated monooxygenase activities. The incubation mixture contained 0.1 M potassium phosphate buffer (pH 7.4), cocktail probe substrates, S9 fraction (20 μL), and a NADPH-generating system (NGS) containing 0.1M glucose 6-phosphate, 10 mg/mL
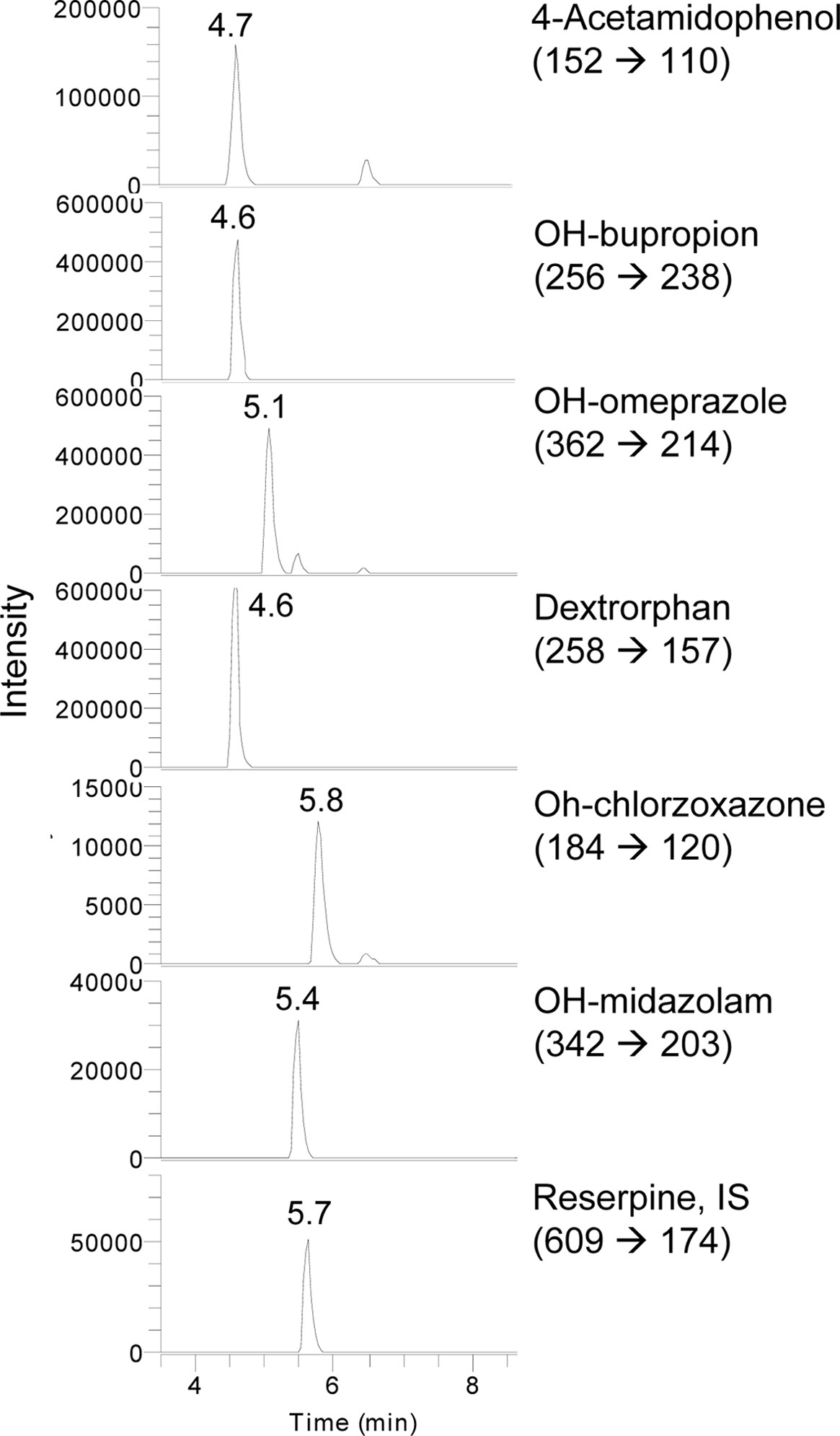
Assays of CYP enzyme activity were performed using an Accela™ LC system coupled to a TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher Scientific Inc., USA) equipped with a HESI-II Spray source. The instrument was operated in selective reaction monitoring (SRM) mode. Electrospray ionization was performed in positive ion mode at a spray voltage of 3,500 kV. Nitrogen was used as the sheath and auxiliary gas with optimum values of 60 and 20 (arbitrary units), respectively. The vaporizer and capillary temperatures were 150 and 300 ℃, respectively. Mobile phases consisted of 0.1% formic acid (A) and acetonitrile (B). The initial composition was increased to 90% solvent (B) for 15 min. A gradient program with a flow rate of 230 μL/min was used for HPLC separation.
Results are expressed as mean values ± standard errors (SE). Dunnett’s t-test was used to determine the significances of differences. Statistical significance was accepted for p values of < 0.01 (abbreviated to **).
In our previous study, mollugin was found to inhibit the activity of hepatic CYP1A2 in pooled HLMs selectively
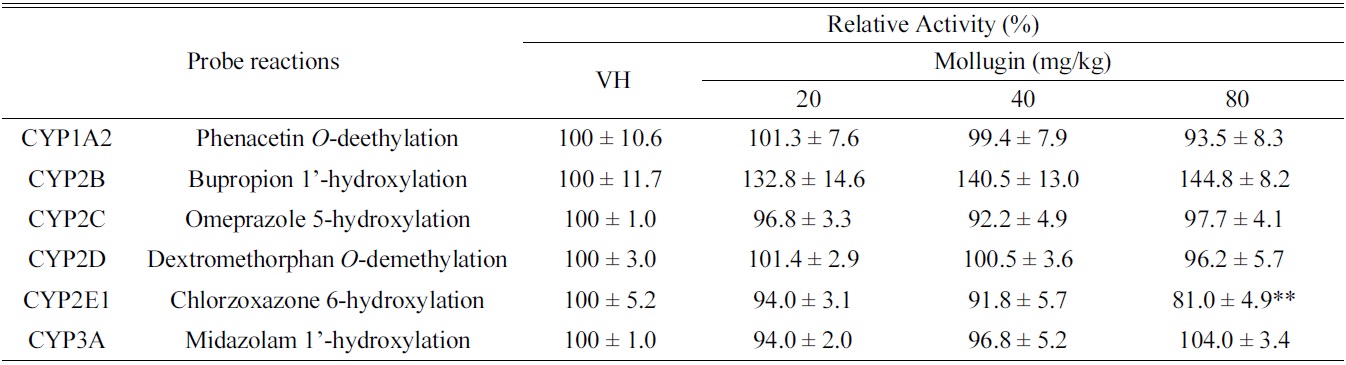
[Table 1.] Effects of oral mollugin on the activities of hepatic CYPs in male ICR mice.
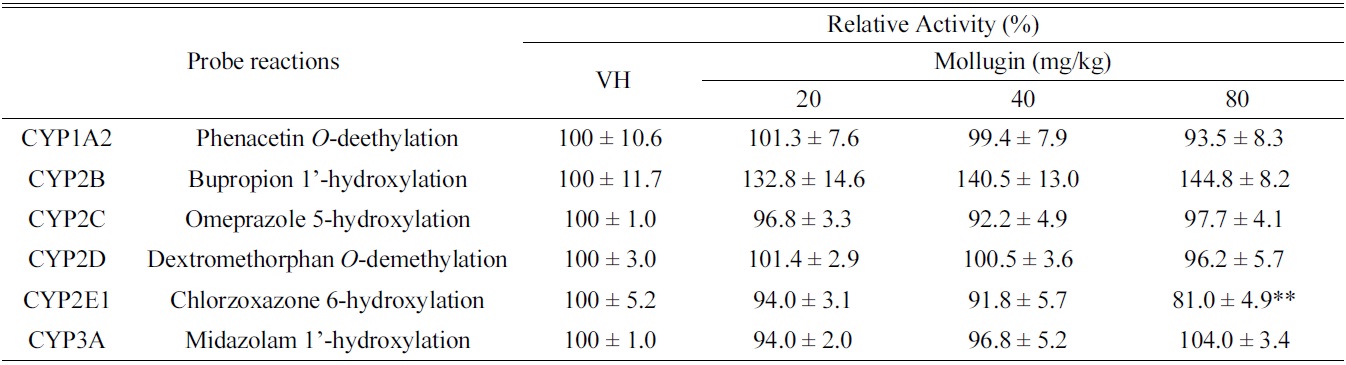
Effects of oral mollugin on the activities of hepatic CYPs in male ICR mice.
and in a competitive manner.14 However, usually the
In this study, we adapted the specific substrates for human CYP isoforms to investigate the activities of mice CYP isoforms. The enzyme activities were determined by incubation with hepatic S-9 fraction instead of microsomal fraction, nevertheless the activities of six CYP isoforms were successfully determined coupling with LC-MS/MS analysis. The investigation of activity of hepatic CYP in mice is important technology to predict the drug-drug interaction or modulatory effects by treatment of chemicals. Therefore, based on confirmed chemical probe assay coupled with LCMS/MS analysis, we could conduct to investigate the modulatory effects after treatment of mollugin
As shown in Table 1, mollugin significantly inhibited the activity of CYP2E1-catalyzed chlorzoxazone 6-hydroxylase in mice hepatic S-9 fractions. On the other hand, other CYP-catalyzed reactions were not significantly changed by mollugin. In particular,non-significantly inhibited the activity of CYP1A2-catalyzed phenacetin
In the present study, we investigated the modulatory effects of mollugin orally administered at 20, 40, or 80 mg/ kg daily for 3 days to male ICR mice in hepatic S9 fractions. Although CYP2E1-catalyzed chlorzoxazone 6-hydroxylation was significantly inhibited by mollugin, others hepatic CYP activities were unchanged. These results suggest that mollugin possibly modulates CYP activities