Monitoring of subcutaneous blood flow is of high importance in fundamental studies, clinical diagnoses, and intraoperative procedures because it provides critical information on the condition of biological tissues and the various disorders in human organs [1,2].
Optical methods such as laser Doppler flowmetry (LDF) and laser Doppler imaging (LDI) have been widely used in clinics to measure tissue blood perfusion and to detect vasculature with minimal invasiveness [3]. LDF has been typically used to monitor relative changes in blood flow with temporal resolution. However, LDF is based on a point measurement method that measures only a specific site of the entire vasculature. Although LDI provides spatial information, it requires a long scanning time to obtain spatially resolved images of relative changes in blood flow. Therefore, its temporal resolution is insufficient to image the changes in blood flow. The trade-off between spatial and temporal resolutions limits the use of laser Doppler methods in many clinical and research applications [2,4-6].
Laser speckle contrast image (LSCI) provides two-dimensional (2D) image of blood flow velocity in biological tissue and has been widely used because of its simplicity, real-time feedback, and negligible motion artifacts [3,5]. In addition, the LSCI provides a noninvasive full-field image and has no trade-off between spatial and temporal resolutions. The LSCI has been utilized for various studies such as peripheral arterial occlusive disease, blood flow in the cerebrum and cortex, retinal optic nerve disorders, blood flow in nearsurface tissues such as skin and mesenteric microcirculation, and
Various optical and image processing methods have been proposed for enhancing the contrast of LSCI in subcutaneous blood flow measurements [1,8-13]. However, LSCI still suffers from low image contrast, although the limitation of laser penetration depth in tissue due to the light scattering property has been partially addressed. In this study, a physicochemical tissue optical clearing (PCTOC) method [14] that is effective in the reduction of light scattering in tissue was applied to enhance the contrast of LSCI of vasculature in deep tissue layers, which is not clearly observed by the naked eye.
2.1. Principle of Laser Speckle Contrast Imaging
Speckle is a random interference pattern produced by fluctuations in the spatial or temporal intensity of laser scattered from the illuminated surface. When the illuminated particles move, the interference pattern changes over time, and finally, a time-varying speckle pattern is generated. The LSCI is used to statistically analyze the intensity fluctuations of the time-varying speckle pattern and provides 2D velocity information about moving particles with spatial and temporal resolutions.
The intensity variation is quantified by local speckle contrast and is defined as the ratio of the standard deviation to the mean intensity of the speckle image [15].
A window of N × N pixels is moved to compute the local speckle contrast along the image. The window size is typically chosen by considering the trade-off between statistical accuracy and spatial resolution. A small window size reduces the statistical accuracy, whereas a larger window size limits the spatial resolution. This study used a 5 × 5 pixel window.
To ensure proper sampling for the LSCI, it is necessary that the size of a single speckle in the imaging plane approximately equals the size of a single pixel in the chargecoupled device (CCD) camera. The speckle size is determined as follows [3]:
where
2.2. Laser Speckle Contrast Imaging Modality
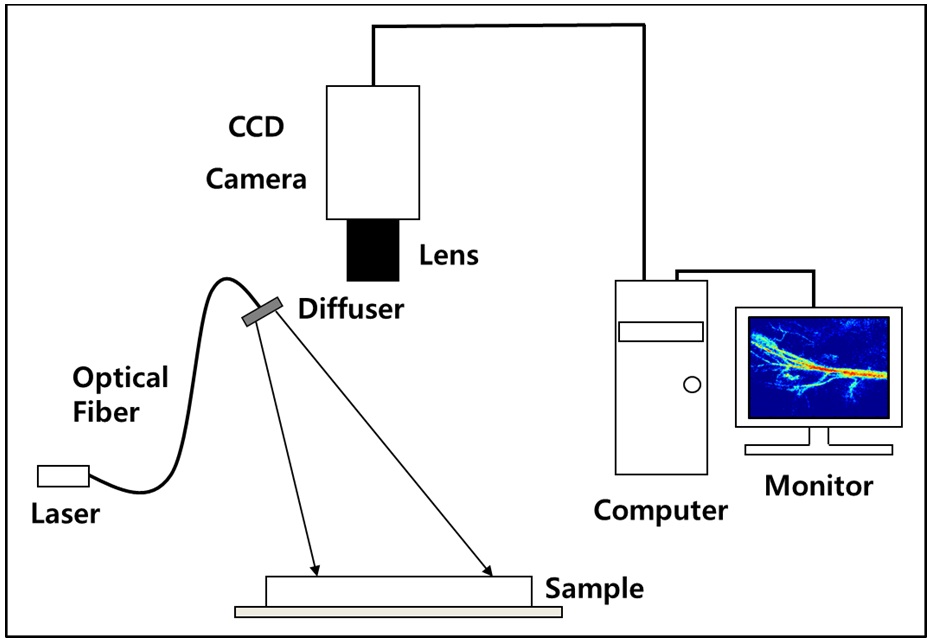
Figure 1 shows the schematic diagram of the experimental setup. A diode laser (HL6512MG; 658 nm, 50 mW; Thorlabs, Newton, NJ, USA) was coupled into an optical fiber. A holographic diffuser was mounted in front of the optical fiber to evenly illuminate the region of interest (ROI). The ROI was imaged with a monochromatic progressive
scan CCD camera (XC-HR57; Sony, Tokyo, Japan) with a resolution of 648 × 494 pixels.
For the
A PCTOC method [14], which combines the microneedling method and the topical application of glycerol, was applied to
In the
2.6. Quantitative Evaluation of LSIC
The PCTOC enhances light penetration depth by reducing light scattering in tissue and therefore, revealing more vasculature in deep tissue layer. As a result, LSCI provides more clear blood flow information in vasculature. Such an effect was quantitatively evaluated by computing relative speckle index (RSI) from the
In the
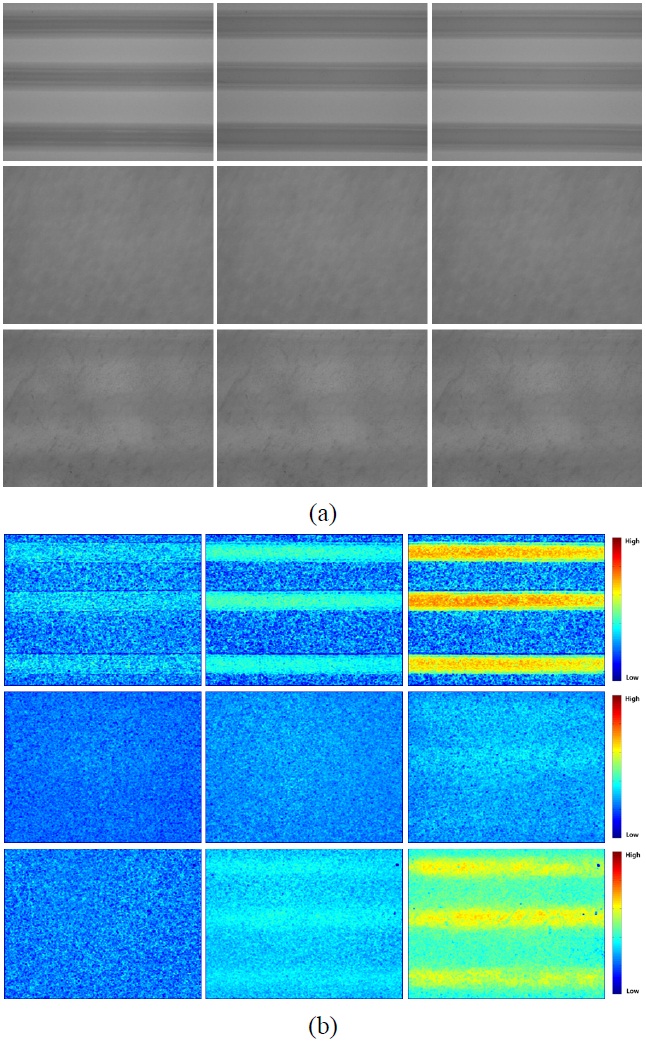
Figure 2(a) shows the change in tissue turbidity that resulted from applying the PCTOC to
Figure 2(b) shows the
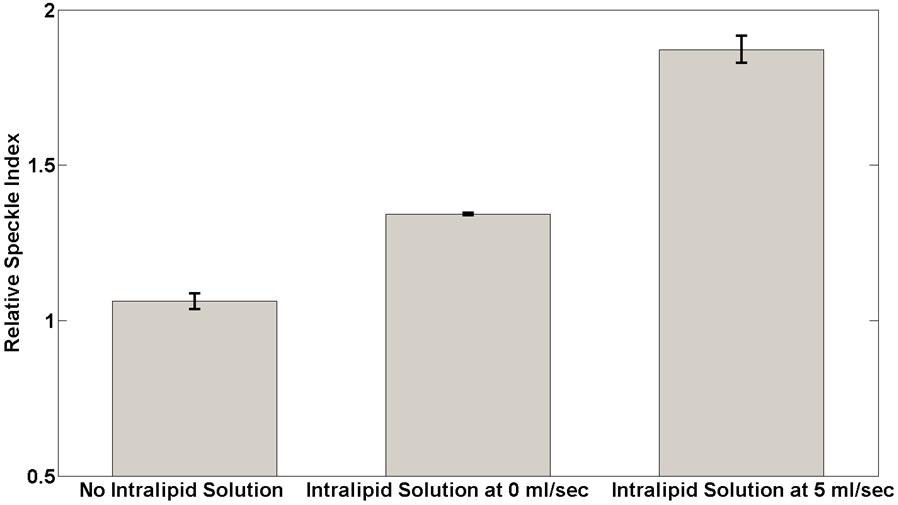
Figure 3 shows quantitative analysis of RSI changes in the LSCI as calculated from the middle and bottom images in Fig. 2(b) using equation (3). The RSI increased after the application of the PCTOC and as a function of flow velocity of the intralipid solution. The RSI increased by 6.18, 34.18, and 87.15% for the cases without the intralipid solution inside the tubing, and with intralipid solution inside the tubing at 0 and 5 ml/sec, respectively.
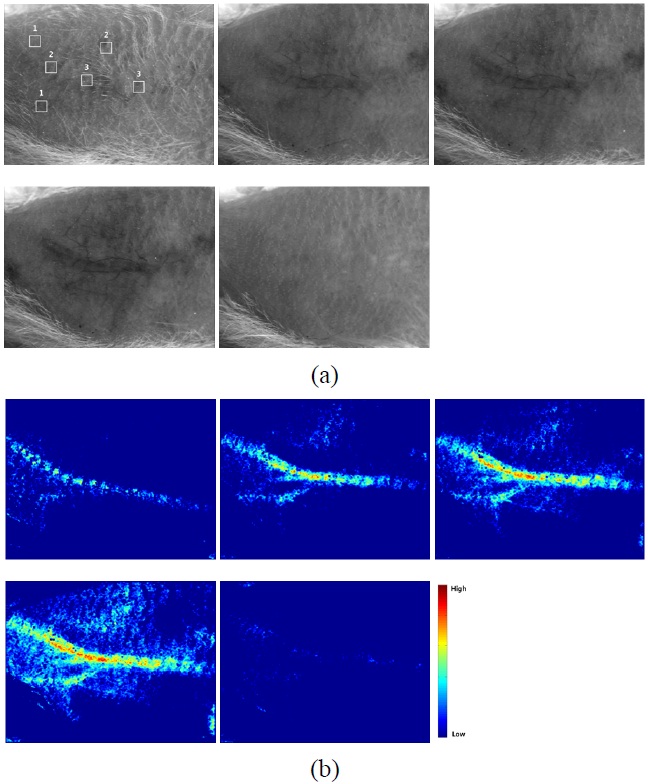
The subcutaneous vasculature, which is not clearly visible to the naked eye, became visible after the application of the PCTOC (Fig. 4(a)). The vasculature was clearly observed 30 min after the application of the PCTOC and returned to normal 30 min after the application of the saline solution.
Figure 4(b) shows the LSCIs of Fig. 4(a). The LSCI
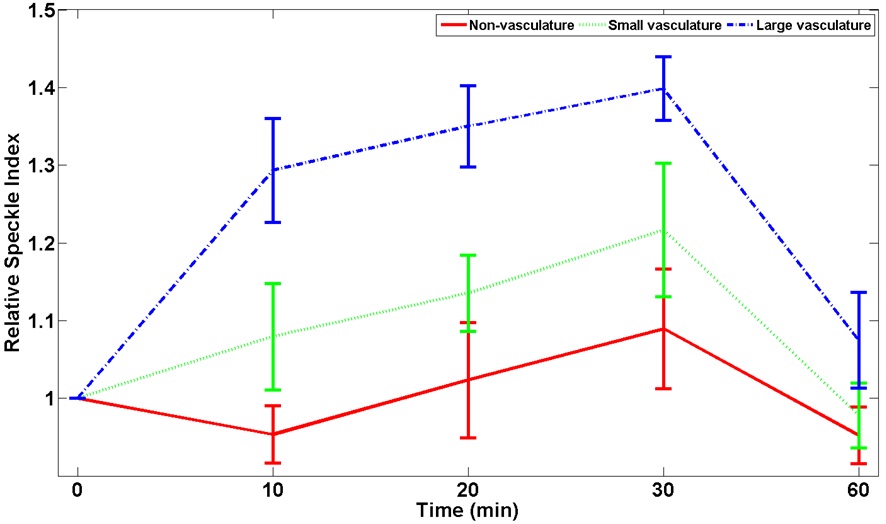
increased as a function of time after the application of the PCTOC and decreased after the application of the saline solution. Fig. 5 shows the quantitative analysis of RSI as a function of time for nonvasculature, small, and large vasculature regions in three
The PCTOC effects were evaluated by observing the tubing structure under the
Most of the previous studies focused on the observation of
As shown in Figs. 2(b) and 3, the LSCI depends on the application of the intralipid solution. Without the application of the PCTOC, the LSCI did not adequately reflect the flow information of the intralipid solution in the tubing (middle images in Fig. 2(b)). However, the LSCI and RSI increased after the application of the PCTOC (bottom images in Figs. 2(b) and (3)). When the intralipid solution was inside the tubing, the LSCI somewhat increased even at 0 ml/sec after the application of the PCTOC. It may be because the stationary intralipid solution slowly moves and scatters the illuminating laser light. The tubing structures were distinguishable and the flow information of the intralipid solution can be verified due to the enhanced LSCI. The tubing region shown in the bottom images in Fig. 2(b) exhibits an LSCI similar to that of the top images, but the surrounding region of the tubing shows an increased LSCI (bottom center and right images). This may be owing to the reduction in the tissue turbidity by the PCTOC, which enhances the LSCI in not only the tubing region but also the surrounding region of the tubing.
As shown in Fig. 4(b), the LSCI increased as a function of time and the femoral artery gradually exhibited faster blood flow, showing more regions of small and large vasculature, which indicates a reduction in tissue turbidity. The LSCI changed more rapidly in large vasculature than in small vasculature because more red blood cells may reflect better LSCI [3], and increased as a function of time after the application of the PCTOC because of a reduction in tissue turbidity. The nonvasculature region did not show significant changes, because no blood flow information was reflected to the LSCI. When saline solution was applied to a transparent skin region, the LSCI was recovered to a normal skin condition because the transparent skin may become turbid again by the saline solution [16].
In general, subcutaneous blood flow, which is a dynamically fluctuating biological variable and has substantial spatial heterogeneity, is invasively monitored by performing open surgery to reveal vasculature [1,5,7,8,11]. However, it was successfully monitored in real time in the present study without open surgery by applying the PCTOC, which clearly reveals vasculature in deep tissue layers.
The LSCI combined with the PCTOC was greatly enhanced even in deep vasculature. The LSCI increased as a function of time after the application of the PCTOC and was properly recovered to initial conditions after the application of a saline solution. It is expected that similar results may be obtained using the suggested LSCI in