Endemism and vulnerability are two important sides of island biodiversity. Island organisms show peculiar morphologies (Millien, 2006) and genetic properties (Sato et al., 2009b), while small populations on islands with such endemism tend to be fragile due to lower genetic potential and/or environmental stochasticity (Frankham et al., 2010). Kier et al. (2009) assessed endemism richness of plants and vertebrates worldwide and clarified that the island biota is more endemic and susceptible to the human impact than the continental ones. Species facing imminent extinction was shown to be concentrated on island biota (Ricketts et al., 2005). Islands can therefore appropriately be called “biodiversity and extinction hotspots.” Understanding the mechanisms for the gain and loss of the biodiversity of the islands is imperative for the conservation of the endangered species that is being lost on islands.
The Japanese archipelagos consist of more than 6,800 islands. They are usually arranged into three biogeographic regions shown on Fig. 1: Hokkaido, Honshu-Shikoku-Kyushu, and Ryukyu (Kawamura, 1991, 1994; Dobson, 1994; Dobson and Kawamura, 1998). The major islands, which are supposed to have been repeatedly connected to the Asian continent during the Pleistocene glacial periods, have received influxes of continental organisms and isolated them differentially in each biogeographic region (Dobson, 1994; Millien-Parra and Jaeger, 1999), leading to various levels of endemic organisms among major islands (Suzuki, 2009). Compared with the same
[Fig. 1.] A diagram for configurations of islands around the Japanese archipelagos (Sakhalin [Karafuto], Hokkaido, Honshu, Shikoku, Kyushu, Tsushima, Ryukyu, Jeju, and Taiwan Islands) and major straits (Tatar [Mamiya], La Perouse [Soya], Tsugaru, Tsushima, Korean, Tokara, and Kerama Straits).
latitudinal regions, the Japanese archipelagos have rich endemic species, especially in mammalian fauna (Kier et al., 2009). The Japanese islands provide an excellent opportunity for understanding the mechanisms for the generation of the biodiversity.
In the modern conservation framework, evolutionary distinctiveness is considered useful for the conservation prioritization (Isaac et al., 2007). Evolutionary history should be clarified to know what should be conserved first (Collen et al., 2011). In addition, adaptive ecological characteristics should also be considered in conservation biology (Ryder, 1986; de Guia and Saitoh, 2007; Isaac et al., 2007; Allendorf et al., 2010; Collen et al., 2011; Funk et al., 2012). It is therefore of great importance to clarify the contribution of both phylogenetic and ecological effects to the formation of species assemblages underpinning regional biodiversity (Webb et al., 2002; Wiens and Donoghue, 2004; Wiens and Graham, 2005).
This review summarizes evolutionary histories and food habits of mustelid carnivoran species in Japan, and assesses the historical and ecological causes for the community assemblage of mustelid fauna in the different biogeographic regions.
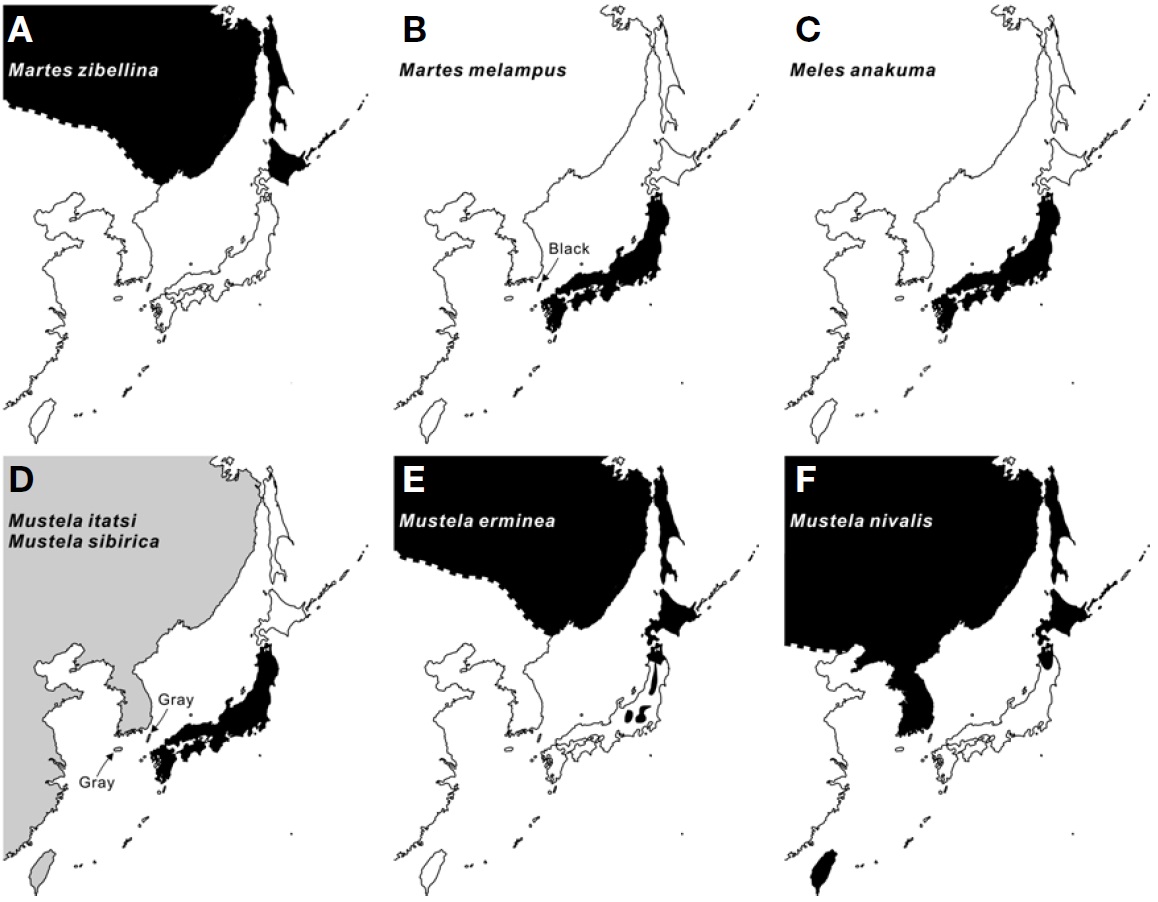
There are seven extant and indigenous terrestrial mustelid species in Japan (Ohdachi et al., 2009), the Japanese marten Martes melampus (Wagner, 1840), the sable Martes zibellina (Linnaeus, 1758), the Japanese badger Meles anakuma Temminck, 1844, the ermine or the stoat Mustela erminea Linnaeus, 1758, the Japanese weasel Mustela itatsi Temminck, 1844, the least weasel Mustela nivalis Linnaeus, 1766, and the Siberian weasel Mustela sibirica Pallas, 1773. The natural distributions of these seven species (Fig. 2) can be summarized into four biogeographic patterns: 1) present only in Hokkaido in Japan with the same species expanded to the continental Palaearctic region (Ma. zibellina) (Fig. 2A); 2) endemic to the Honshu-Shikoku-Kyushu region (Ma. melampus, Mu. itatsi, and Me. anakuma) (Fig. 2B-D); 3) present only in Tsushima Islands (Mu. sibirica) (Fig. 2D); and 4) Present in Hokkaido and a part of the northern Honshu in
Japan with the same species expanded to the Holarctic region (Mu. erminea and Mu. nivalis) (Fig. 2E, F). Recent extensive accumulation of data on evolutionary and feeding ecological studies would enable clarification of the historical and ecological meaning of the distributions.
I first reviewed the molecular evolutionary and feeding ecological studies on mustelids in Japan, and then discuss the faunal assemblage of the Japanese mustelid species. Two species below were not included in this review. The American mink Neovison vison (Schreber, 1777) is considered an exotic species from North America (Uraguchi, 2009). The Eurasian otter Lutra lutra (Linnaeus, 1758), an aquatic mustelid, is currently recognized as an extinct species (Sasaki, 2009b) and has provided too few phylogeographic and ecological data to review. In addition, I do not discuss the artificially introduced populations (e.g., Ma. melampus in Hokkaido and Sado Islands, Mu. itatsi in Hokkaido Islands, and Mu. sibirica in the western part of the Honshu Islands) to assess the establishment process of the natural distribution.
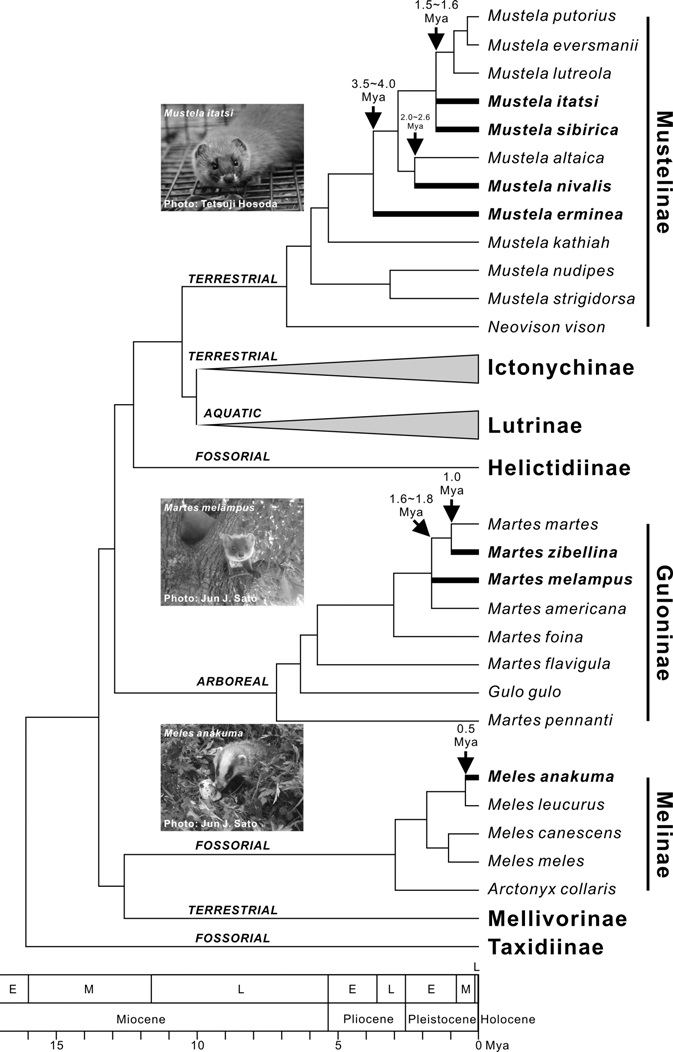
Martes zibellina. Recent molecular phylogenetic studies demonstrated that Ma. zibellina is closely related to the pine marten Martes martes (Linnaeus, 1758) (e.g., Koepfli et al., 2008; Sato et al., 2012) (Fig. 3). The divergence between them was estimated to have occurred approximately 1.0 million years ago (Mya) by chronological analyses using 22 nuclear and mitochondrial genes (Koepfli et al., 2008) (Fig. 3). Among intraspecific variations, the sable in Hokkaido is classified as a local subspecies, Martes zibellina brachyura (Temmink, 1844), based on morphology (Anderson, 1970; Wozencraft, 2005). Consistent with the morphological data,
[Fig. 3.] Interspecific phylogenetic relationships and divergence times of lineages within the family Mustelidae reconstructed on the basis of recent molecular phylogenetic studies (Sato et al., 2003, 2004, 2006, 2009a, 2012; Koepfli et al., 2008; Wolsan and Sato, 2010; Tashima et al., 2011). I followed Wolsan and Sato (2010) and Sato et al. (2012) for the subfamilial designation. Branches and scientific names for the Japanese mustelid species were highlighted with thick line and bold font, respectively. I assigned ecological types (arboreal, aquatic, fossorial, and terrestrial) to each subfamily according to Nowak (1999), although some exceptions exist (e.g., semi-aquatic Neovison vison in Mustelinae). Mya, million years ago.
Sato et al. (2011) examining the mitochondrial NADH dehydrogenese subunit 2 (Nd2) gene sequences indicated that the Hokkaido sable possessed endemic genetic property. The analyses of the mitochondrial cytochrome b (Cytb) gene also supported the distinctive nature of the Japanese sable (Hosoda et al., 1999, 2000; Ishida et al., 2013). Sato et al. (2011) estimated the migration time of Ma. zibellina in Hokkaido to be 0.27-0.10 Mya, but stressed that 0.10 Mya would be closer to the migration date by considering the geological evidence. McKay (2012) re-examined the Cytb data of Hosoda et al. (2000) and provided 0.08 Mya as divergence time for Hokkaido and the continental populations. More recently, Ishida et al. (2013) examined two closely linked nuclear genes, melanocortin 1 receptor (Mc1r) and transcription factor 25 (Tcf25), and inferred the migration time to be ca. 0.05-0.01 Mya by using the recombination rate between these two genes. Although the time estimates above differed from each other, they consistently showed that the migration of this species from the continent could have occurred in the Late Pleistocene (0.13-0.01 Mya).
Martes melampus. Although Kuroda and Mori (1923) reported the existence of this species in South Korea and some studies have provided corroboration (e.g., Wozencraft, 2005; Monakhov, 2011), I presently suppose that this species is endemic to Japan according to a recent illustrated reference book (Ohdachi et al., 2009). Previous interspecific systematic studies have clarified the species level endemism of this species, but failed to resolve the phylogenetic relationships among Ma. melampus, the American marten Martes americana (Turton, 1806), and a clade including Ma. martes and Ma. zibellina (Koepfli et al., 2008; Sato et al., 2012) (Fig. 3). Nevertheless, they consistently showed that the lineage of Ma. melampus was earlier generated than Ma. zibellina. The divergence time of Ma. melampus from the other Martes species was estimated to be 1.6-1.8 Mya (Koepfli et al., 2008; Sato et al., 2012) (Fig. 3). McKay (2012) re-examined the Cytb data of Hosoda et al. (2000) and showed a relatively younger estimate (1.36 Mya). It has not been clearly understood whether the ancestral lineage of Ma. melampus migrated from northern Sakhalin or southern Korean Peninsula. However, considering the current circumboreal distribution of the Holarctic martens in Eurasia, the migration through northern route via Sakhalin seems plausible. Recently Ishida et al. (2013) found some Mc1r and Tcf25 gene haplotypes from Ma. zibellina in Hokkaido that were closely related to those of Ma. americana and Ma. melampus. They might be considered remains of ancestral populations in Hokkaido leading to the extant Japanese marten lineages in Honshu Islands (Ishida et al., 2013).
For intraspecific diversity of Ma. melampus, Kurose et al. (1999) and Inoue et al. (2010) did not detect any correlations between sampling locations and genetic variations in the mitochondrial Cytb and Dloop sequences, respectively. On the other hand, Sato et al. (2009b), by using more diverse data from three mitochondrial genetic loci (Cytb, Nd2, and Dloop), reported some extent of geographic structures within this species, showing distinct genetic property of each Tohoku, Tochigi-Niigata, Kyushu, Tsushima, and the other western Japanese lineage. To date, no studies have introduced time axis for intraspecific diversity of this species. Here, I simply calculated the within-species divergence times on the basis of the molecular clock assumption and 1.6-1.8 million years for the Ma. melampus-Ma. zibellina split, using data of Sato et al. (2009b). The result showed that the estimated time to the most recent common ancestor of the total examined individuals for Ma. melampus in Sato et al. (2009b) was 0.32- 0.36 Mya, indicating that the divergence events within this species might have occurred in the Middle Pleistocene (0.78- 0.13 Mya).
Meles anakuma. The Japanese badger was once a subspecies of the Eurasian badger Meles meles (Linnaeus, 1758) (e.g., Wozencraft, 1993). However recent molecular phylogenetic studies have demonstrated that it has a distinctive evolutionary lineage from European and the other Asian continental badgers (Marmi et al., 2006; Cerro et al., 2010; Tashima et al., 2011). It is now regarded as a distinct species within the genus Meles together with European Me. meles and North and East Asian Meles leucurus (Hodgson, 1847) (see Wozencraft, 2005). Recently Cerro et al. (2010) introduced an additional Meles species, Me. canescens, from Southwest Asia on the basis of the genetic variations in six nuclear and one mitochondrial gene sequences. The molecular systematic conclusion is consistent with considerable geographic variations in morphology such as facial colour patterns and penis bone structures across the Eurasian continent and Japan (Abramov, 2002, 2003). Meles anakuma is the most closely related to Me. leucurus, and the divergence between them was estimated to have occurred 0.5 Mya (Tashima et al., 2011) (Fig. 3). The time estimate is in agreement with the fossil records, suggesting that the immigration through Korean Peninsula occurred in the middle Middle Pleistocene period (0.43 Mya) (Ogino et al., 2009). Kaneko (2009) supposed on the basis of the absence of this species in Hokkaido and Ryukyu Islands that an ancestral lineage of Me. anakuma migrated via Korean Peninsula. It should be noted that McKay (2012) re-examined the Cytb data of Kurose et al. (2001) and provided an older estimate (1.4 Mya) for the divergence time between Me. anakuma and Me. leucurus, which is inconsistent with the aforementioned molecular and fossil evidence (Ogino et al., 2009; Tashima et al., 2011).
Kurose et al. (2001) showed very low intraspecific genetic diversity in the Cytb gene, and attributed the result to the recent population expansion. Tashima et al. (2011) supported Kurose et al. (2001) for the recent expansion by showing negative Tajima’s D (Tajima, 1989) and Fu’s Fs (Fu, 1997) values using the Dloop sequences. The population expansion was suggested to have begun 0.23-0.11 Mya (Tashima et al., 2011).
Mustela itatsi. Although previously the Japanese weasel was classified as a subspecies of the Siberian weasel Mustela sibirica (e.g., Wozencraft, 1993), interspecific level of distinctiveness of this species has been supported by the recent molecular phylogenetic (e.g., Masuda and Yoshida, 1994; Sato et al., 2003, 2012) and morphological (Suzuki et al., 2011) studies. The Japanese weasel currently has a valid species name, Mustela itatsi (Wozencraft, 2005). Phylogenetic position of this species has remained to be elucidated. Sato et al. (2012) examined 10 genetic loci (nine nuclear and one mitochondrial genes), but failed to resolve the phylogenetic relationships among Mu. itatsi, Mu. sibirica, and the clade encompassing the European mustelids, the steppe polecat Mustela eversmanii Lesson, 1827, the European mink Mustela lutreola (Linnaeus, 1761), and the European polecat Mustela putorius Linnaeus, 1758 (Fig. 3). The molecular chronological analyses in Sato et al. (2009a, 2012) showed that the time for the trichotomous divergence among those three lineages was 1.5-1.6 Mya (Fig. 3). Although it is not clear when the lineage of Mu. itatsi placed its origin in Japan, Ogino et al. (2009) showed on the basis of the fossil evidence that Mu. itatsi was present in Kyushu Islands in the middle Middle Pleistocene period (0.43 Mya) at the latest. McKay (2012) re-examined the Cytb data of Hosoda et al. (2000) and Marmi et al. (2004), and showed more ancient divergence between Mu. itatsi and Mu. sibirica (2.88 Mya), which seems an overestimate compared with the molecular estimate from nine nuclear and one mitochondrial gene sequences (Sato et al., 2009a, 2012) and fossil evidence (Ogino et al., 2009).
Few studies have contributed to the intraspecific genetic diversity of this species, but recently Masuda et al. (2012) summarized its phylogeographic history. By using the Dloop sequence variations, they clarified two distinct evolutionary lineages of the Japanese weasel, Honshu and Kyushu-Shikoku lineages. The divergence time of these two lineages was estimated to be 0.83-1.17 Mya. Their demographic analyses captured the tendency of the recent expansion of the Honshu population that occurred 0.54 or 0.77 Mya. On the other hand, the Kyushu-Shikoku population appeared to have more stable population history within refugia in the Pleistocene glacial periods, and the population expansion (albeit not clearly indicated) was inferred to have occurred 0.59 or 0.83 Mya.
Mustela sibirica. The Siberian weasel is only observed in Tsushima Islands in Japan, providing a peculiar biogeographic pattern in the Japanese mustelid fauna, although descendants of the populations artificially introduced in the early 20th century are currently expanded to the western part of Japan (Sasaki, 2009a). As described above, the lineage of the Siberian weasel, Mu. sibirica, originally occurred in the early Pleistocene at the same time with that of the Japanese weasel, Mu. itatsi (1.5-1.6 Mya) (Fig. 3) (Sato et al., 2012).
On the other hand, the migration time to the Tsushima Islands seems to be very recent because Mu. sibirica in Tsushima Islands was demonstrated to have the same Cytb gene haplotype (402 bp) as that observed in Korea (Hosoda et al., 2000). Meanwhile, Masuda et al. (2012) showed the genetic differentiations of the Dloop haplotypes observed in the Tsushima and Taiwan Islands from the continental Russian and Korean haplotypes. Since they did not infer the divergence times between these continental and island haplotypes, the chronology for the origin of the Tsushima populations has remained to be enlightened.
Mustela erminea. Phylogenetic analyses have indicated an origin of the lineage of Mustela erminea in the Early to Late Pliocene (3.5-4.0 Mya) (Fig. 3) (Koepfli et al., 2008; Sato et al., 2009a, 2012). In contrast to the relatively old origin compared with the other terrestrial mustelid species living in Japan, mitochondrial Dloop sequence analyses showed low intraspecific variations, suggesting recent diversifications across the Eurasian continent (Kurose et al., 2005). Meanwhile, Kurose et al. (2005) clarified that Mu. erminea in Japan had two distinct lineages, in which the clade including three individuals from both Honshu and Hokkaido showed close affinity to the North American individuals, while one Honshu individual was more closely related to a continental Russian individual than the other Japanese ones, implying multiple migrations into the Japanese archipelagos. Although time scale for intraspecific divergence has not been precisely estimated, fossil records in Kawamura et al. (1989) showed that Mu. erminea was present in the middle and late Middle Pleistocene in Yamaguchi prefecture (southern Honshu), and in the early Late Pleistocene in the Tochigi prefecture (central Honshu).
Mustela nivalis. Mustela nivalis showed close phylogenetic affinity to the mountain weasel Mustela altaica Pallas, 1811, and divergence time between the lineages of these species was estimated to be 2.0-2.6 Mya in Late Pliocene to Early Pleistocene (Fig. 3) (Koepfli et al., 2008; Sato et al., 2009a, 2012). Although Mu. nivalis has a similar distribution pattern to Mu. erminea, intraspecific diversity is much more extensive for Mu. nivalis in terms of both morphological (Abramov and Baryshnikov, 2000) and genetic (Kurose et al., 2005) characteristics. As in Mu. erminea, Kurose et al. (2005) found two distinct lineages in Japan (Honshu and Hokkaido lineages), similarly requiring multiple migrations for their distributions. Since the karyotypes of the Honshu and Hokkaido populations differ from each other (2n=38 and 42, respectively), Obara (1991) proposed that the Honshu population be treated as distinct species, Mustela namiyei Kuroda, 1921. However, the level of the difference between Honshu and Hokkaido populations is currently considered an intraspecific variation (Hosoda et al., 2000; Kurose et al., 2005). To date, there have been no chronological analyses for the migrations into the Japanese archipelagos. Thus, the fossil discovered in the late Late Pleistocene in Gifu prefecture (central Honshu) is the only valuable chronological evidence (Kawamura et al., 1989).
The sable is an arboreal carnivoran species in the genus Martes, occupying both coniferous and deciduous forests across northern Eurasia and Hokkaido, Japan (Murakami,
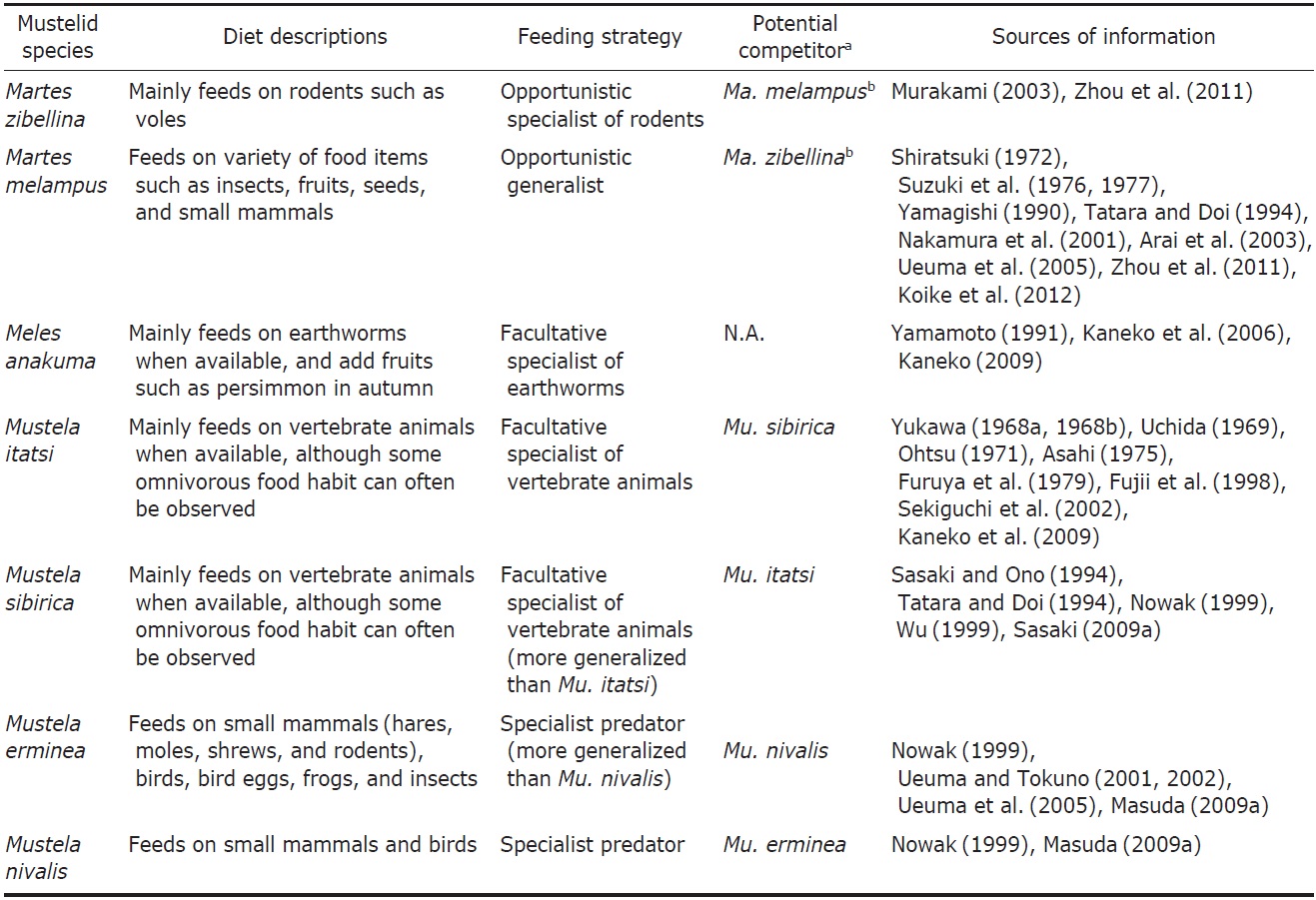
2009). The sable largely depends on these forest resources. Martes species is usually considered an opportunistic generalist, whose diet changes depending on the environments they inhabit (Zhou et al., 2011). Meanwhile, the prey of Ma. zibellina is relatively biased to small mammals such as rodents (Zhou et al., 2011). Murakami (2003) examined food habits of the Japanese sable, and clarified that the sable preyed mainly on mammals like voles throughout the year, although it showed omnivorous nature depending on the availability of food items in different seasons. The sable can be considered an opportunistic specialist of rodents (Table 1).
There is a tendency of Martes species to prey on mammals such as rodents in the colder northern region, while they eat more invertebrates and plants in warmer southern region (Zhou et al., 2011). Concordantly, Ma. melampus, an inhabitant of the southern Japanese islands, consumes more insects and fruits in its diet in addition to small mammals than most other martens (Shiratsuki, 1972; Suzuki et al., 1976, 1977; Yamagishi, 1990; Tatara and Doi, 1994; Nakamura et al., 2001; Arai et al., 2003; Ueuma et al., 2005; Zhou et al., 2011; Koike et al., 2012). This species is well known as a seed disperser of many plant species (Otani, 2002; Tsuji et al., 2011). Around suburban areas of Tokyo, seeds, fruits, and insects dominate as the main items in its fecal composition (Nakamura et al., 2001). In the Tsushima Islands, Ma. melampus preys on insects, centipedes, and plants more frequently than sympatric carnivores, the Siberian weasel Mu. sibirica and the leopard cat Prionailurus bengalensis (Kerr, 1792) (see Tatara and Doi, 1994). Ma. melampus tends to show very high diversity of food items, representing an opportunistic generalist property (Tatara and Doi, 1994; Yamamoto, 1994) (Table 1). Arai et al. (2003) mentioned that a wide variety of food items and flexible feeding strategy of Ma. melampus suggested high potential of adaptability to various food environments.
Badgers in the genus Meles are a morphologically and ecologically distinct form within the family Mustelidae, which have adapted to a fossorial life-style (Fig. 3) (Kaneko, 2009). The diet of Me. anakuma concentrates on earthworms from spring to autumn, although it also prefers fruits such as the persimmon in autumn (Yamamoto, 1991; Kaneko et al., 2006; Kaneko, 2009). Me. anakuma in Mt. Nyugasa (Nagano Prefecture) has the least diverse diet, being concentrated on earthworms and insects, compared with the other sympatric carnivores, the Japanese marten Ma. melampus, the red fox Vulpes vulpes (Linnaeus, 1758), and the raccoon dog Nyctereutes procyonoides (Gray, 1834) (see Yamamoto, 1994). Thus, Me. anakuma may be viewed as a facultative specialist of earthworms (a vermivore), although overall it is a generalist (Table 1). The diet diversity of the European badger Me. meles was shown to be negatively correlated with earthworm volume in the diet, assisting a facultative earthworm specialist property of “badgers” (Virgos et al., 2004; but see Roper, 1994 for the opportunistic generalist badger hypothesis).
Because the Japanese weasel prefers wild rodents as a prey, it plays an important role as a biological pest control species (e.g., Uchida, 1969). Many individuals were released into many islands to suppress the rat density for an agricultural purpose (Masuda and Watanabe, 2009), leading to harmful influence on indigenous biota (Sekiguchi et al., 2002; Hamao et al., 2009). In contrast, previous feeding ecological studies have suggested that Mu. itatsi preys on a variety of vertebrates and invertebrates, and consumes fruits, depending on different seasons or regions (Yukawa, 1968a, 1968b; Uchida, 1969; Ohtsu, 1971; Asahi, 1975; Furuya et al., 1979; Fujii et al., 1998; Sekiguchi et al., 2002; Kaneko et al., 2009). Thus, this species is usually regarded as omnivorous (Masuda and Watanabe, 2009) and capable of adaptation to changes in food availability (Furuya et al., 1979; Fujii et al., 1998; Sekiguchi et al., 2002). However, the diet of this species seem to be mainly dominated by vertebrate animals if present as inferred from the aforementioned studies. Mu. itatsi is considered to be a facultative specialist of vertebrate animals (Table 1).
The Siberian weasel is basically a flesh eater, mainly preying on rodents, shrews, and moles, but is able to adapt to a wide variety of food items including kitchen waste produced by human activities (Sasaki and Ono, 1994; Tatara and Doi, 1994; Nowak, 1999; Wu, 1999; Sasaki, 2009a). This species can be called an opportunistic predator of vertebrate animal and therefore a candidate competitor of the Japanese weasel Mu. itatsi (Table 1), although the former is more generalized to some extent. It is considered that the introduced Siberian weasel in the western Japan is expelling the indigenous Japanese weasel to more mountainous region probably because of the competitions on their ecological niche (Kuroda, 1955; Sasaki, 2009a).
The ermine (or the stoat) is an almost pure-carnivorous species, preying on small mammals (rodents and hares), birds, bird eggs, frogs, and insects (Nowak, 1999; Masuda, 2009a), and representing characteristics of a specialist predator (Table 1). Nevertheless, compared with Mu. nivalis, Mu. erminea has a more diverse diet (Elmeros, 2006). In an alpine habitat, it can even feed on fruits as a supplementary food in summer, probably to reduce the cost of capturing rodents as its preys (Martinoli et al., 2001). Mu. erminea is considered to have a more generalist property only in comparison with Mu. nivalis (King and Moors, 1979). Few studies about the diet of this species in Japan are available, except the studies of Ueuma and Tokuno (2001, 2002) and Ueuma et al. (2005) in Mt. Hakusan located in the nearly southern limit of the distribution in Honshu Islands. The authors showed that, as in continental populations of this species, the diet of Mu. erminea in Japan is mainly dominated by animal prey comprised of shrews, moles, rodents, hares, birds, and insects.
The least weasel is also a nearly pure-carnivorous species (Nowak, 1999; Masuda, 2009b) (Table 1) and has a marked dietary overlap with Mu. erminea (Elmeros, 2006). Despite the overall similarity between Mu. erminea and Mu. nivalis in the prey category concentrated on small mammals and birds, the relative frequencies of their prey types differ; Mu. nivalis eats more small rodents while Mu. erminea adds more rabbits and birds as its main prey (King and Moors, 1979). In addition, these two species select different prey at the species level. For instance, in Denmark, Mu. erminea eats more Microtus voles and watervoles than Mu. nivalis, while Mu. nivalis preys on more bank voles and moles than Mu. erminea (Elmeros, 2006). The differences in food are probably mainly related to prey size, and this also concerns the differences in food of females and males within a species (King, 1989) Usually, Mu. nivalis is considered a more specialized carnivorous predator, exploiting efficiently small rodent preys than Mu. erminea because of its small body size (King and Moors, 1979). The diet of this species in Japan has not yet been thoroughly investigated.
Community assembly and faunal organization could be affected by both phylogeographic and ecological causes. It is quite important to evaluate both ultimate (past historical) and proximate (present ecological) causes for the formation of the species assemblages (Losos, 1996; Webb et al., 2002; Wiens and Donoghue, 2004; Cardillo, 2011). In particular, the Japanese archipelagos possess an intrinsic complicated geo-history, such as different land connection patterns in different ages, and permit diverse ecological niche requirements in various landscapes, such as a long island structure extending from the northern subarctic to the southern subtropical climate zones. By elucidating processes of distributional patterns of mustelid species in Japan with extensive ecological diversity, it is possible to understand how the phylogeography and ecology concertedly contributes to the formation of the species assemblages.
The aforementioned review of up-to-date studies on the origins and population history of the Japanese mustelid species and dietary niche breadth provide information that is useful in understanding the phylogeographic and feeding ecological reasons for the patterns of assemblages by mustelid carnivoran species in Japan. The answers to the following four questions revealed three different constraints that play important roles for the formation of the mustelid species distribution.
Three species endemic to Honshu-Shikoku-Kyushu Islands, Martes melampus, Meles anakuma, and Mustela itatsi, which represent biogeographic pattern II, can be classified into different subfamilies (Fig. 3). Sato et al. (2012) adopted the framework of eight subfamilies within the family Mustelidae on the basis of their well-supported phylogeny, assigning Martes, Meles and Mustela to Guloninae, Melinae, and Mustelinae, respectively (see also Wolsan and Sato, 2010). The family Mustelidae is famous for their extensive ecomorphological diversifications at the subfamilial level (Fig. 3) (Sato et al., 2012; Wolsan, 2013). Species within Guloninae have mainly adapted to an arboreal life style and are adept at climbing trees. The Melinae species has large and strong claws for digging their den (“sett”), which has allowed the group members to adapt to a fossorial life style. The terrestrial Mustelinae species have adapted to more open and waterside habitats. Not surprisingly, dietary niche breadths of three species in different subfamilies are not largely overlapped (Table 1). Although not completely determined, Ma. melampus is a generalist, tasting diverse foods mainly from insects and vegetables, Me. anakuma is basically a specialist of earthworms, and Mu. itatsi mainly feed on vertebrate animals as a specialist (Table 1). Fundamental differences in their diet could have enabled them to co-occur on the Honshu-Shikoku- Kyushu Islands. It was previously implied that the coexistence of ecologically similar bird species was more difficult on islands than on mainland because of more extensive competition for limited resources in the islands (Grant, 1966). Niche heterogeneities could make such coexistence possible in the islands. It could be argued that the co-existence of three species in the Honshu-Shikoku-Kyushu Islands reflect pre-existing ancient ecological differences maintained by their evolutionary niche conservatism (Wiens and Graham, 2005). This is supportive evidence for the deep history hypothesis (present-day species assemblages was established largely due to ancient pre-existing ecological differences) rather than the competition hypothesis (present-day species assemblages was established largely due to ecological differences recently generated by competitions) as a primary cause for enabling the present-day species coexistence (Vitt and Pianka, 2005; also see Wu, 1999). The observation is also consistent with the trend that land-bridge island mammal assemblages tend to be composed of phylogenetically distantly related (overdispersed) lineages (Cardillo et al., 2008).
Grant (1970) found a trend that islands are first occupied by generalists and then by specialists in the study of presence/ absence of some rodents on islands. Piechnik et al. (2008) also supported the generalist-before-specialist colonization hypothesis by analysing the colonization order of arthropods in islands. I tested the hypothesis by evaluating the phylogenetic history of three mustelid species endemic to Honshu- Shikoku-Kyushu Islands. The chronological inference showed that Ma. melampus, Me. anakuma, and Mu. itatsi originated 1.6-1.8, 0.5, and 1.5-1.6 Mya, respectively. Ages for intraspecific diversifications were estimated to be 0.32-0.36, 0.11-0.23, and 0.54-1.17 Mya, respectively. Although it is not clear when the migrations occurred after the time for the generation of the species, these estimates seemingly suggest the earliest colonization by Mu. itatsi, followed by Ma. melampus and Me. anakuma. The generalist-before-specialist hypothesis was therefore not clearly supported. This would probably be because not large overlap in their dietary niche permits any directions of the colonization order. The generalist- before-specialist rule may be applicable to organisms whose primary dietary niche is overlapped or nested, where the colonization of one of them are constrained by the other because of competitions for resources. The Taiwanese mustelids also shows similar tendency that lineages showing basic ecological differences colonized at different ages, independently of the generalist-before-specialist rule, in which the Ferret badgers Melogale moschata (Gray, 1831) in the subfamily Helictidinae migrated from the continent firstly, two Mustela species in Mustelinae secondly, and the Yellow- throated martens Martes flavigula (Boddaert, 1785) in Guloninae in the last (Hosoda et al., 2011). On the other hand, of two congeneric Mustela species in Taiwan whose primary diets such as rodents seem overlapped or nested, Mu. sibirica, a generalist, was inferred to be established earlier than Mu. nivalis, a specialist, which is consistent with the generalistbefore- specialist hypothesis. Also in Japan, two pure-carnivorous specialists, Mu. erminea and Mu. nivalis, seem to have been established later than the congeneric Mu. itatsi which is more generalized than Mu. erminea and Mu. nivalis. Collectively, an important suggestion could be drawn from the first question that evolutionary or phylogenetic constraints (pre-existing ecological differences) would strongly affect the species assemblage.
Martes zibellina is present only in Hokkaido, not in Honshu, Shikoku, Kyushu and Tsushima Islands, whilst the natural distribution of Martes melampus is the reverse to that of Ma. zibellina (Fig. 2A, B), showing an allopatric distribution across the Tsugaru Strait located between Hokkaido and Honshu Islands (also known as Blakiston’s biogeographical line) (Fig. 1). Why is Ma. zibellina absent from southern islands despite of the possibility that Ma. zibellina representing more specialist property could colonize regions where Ma. melampus, a generalist, inhabits (the generalist-beforespecialist rule)? Why is Ma. melampus missing in Hokkaido Islands regardless of the possible ancestral migration from the northern route via Sakhalin and Hokkaido? Addressing these questions could provide us understandings of reasons for the allopatric distribution of two Martes species.
According to the interspecific phylogenetic tree (Fig. 3) (Koepfli et al., 2008; Sato et al., 2012), the generation of the Ma. melampus lineage (1.6-1.8 Mya) occurred earlier than that of Ma. zibellina (ca. 1.0 Mya). It is natural to consider that the lineage of Ma. melampus first migrated into the Japanese archipelagos from the continent and then Ma. zibellina did. Based on the geological survey (Ohshima, 1990), the Tatar (Mamiya) and La Perouse (Soya) Straits (Fig. 1) could have been used as land bridges between the continent and Hokkaido through Sakhalin (Karafuto) in both the Middle (0.78-0.13 Mya) and Late (0.13-0.01 Mya) Pleistocene periods, while the Tsugaru Strait was only available for dispersals in the Middle Pleistocene but not in the Late Pleistocene. This is because decrease in sea level in Late Pleistocene was insufficient for land bridges to be formed in the Tsugaru Strait (Ohshima, 1990). Accordingly, it is possible to predict that Ma. melampus could have established their lineage in Honshu Islands before the onset of the Late Pleistocene, while Ma. zibellina could have migrated into Hokkaido in the Late Pleistocene. The time to the most recent common ancestor of examined individuals of Ma. melampus was estimated to be 0.32-0.36 Mya, which is in agreement with the Middle Pleistocene origin. Although time estimates for the origin of Ma. zibellina in Hokkaido were different in Sato et al. (2011), McKay (2012), and Ishida et al. (2013) adopting different data and chronological methods, they consistently showed that the migration could have occurred in the Late Pleistocene. Those estimates suggest that the arrival of the Ma. zibellina lineage in Hokkaido would have been too late to cross the Tsugaru Strait. This example of the allopatric distribution by two Martes species in Japan implies that “geological constraints” contributed largely to the formation of the species distribution.
The answer for the second question is still not clear. However, Ishida et al. (2013) analyzed two closely linked nuclear genes of Ma. zibellina in Hokkaido and detected traces of ancient hybridization between Ma. zibellina and an ancestral lineage leading to Ma. melampus and Ma. americana, hinting at assimilation of the ancestral genomic components of Ma. melampus in the genome of Ma. zibellina. It is important to perform genome scale analyses for Ma. zibellina in Hokkaido to explore the reasons for disappearance of Ma. melampus linages from Hokkaido. This idea is conditional on the hypothesis that the migration of Ma. melampus via the nouthern route is true. However, the southern route hypothesis would not still be ruled out for the absence of Ma. melampus in Hokkaido.
In spite of the inhabitation by Mustela sibirica in Tsushima Islands which is very closely located to the Japanese main islands, this species is not present in the main islands except the artificially introduced populations (Fig. 2D). Masuda et al. (2012) found specific lineages of Mu. sibirica in the Korean Peninsula, Taiwan, and the Tsushima Islands, and thus proposed the hypothesis that these regions would have played a role as refugia in the Pleistocene glacial periods to generate private mitochondrial DNA lineages. Considering that the current Korean and Tsushima Straits were formed at the similar age (Ohshima, 1990) and assuming that these straits would have probably been used as land bridges in the same glacial period of the Pleistocene, it is curious that Mu. sibirica is absent in the Japanese main islands despite of the presence in Tsushima Islands. Furthermore, the fact that Mu. sibirica is present in the Taiwan Islands increases the mystery. One explanation is that, as Masuda et al. (2012) supposed, the existence of Mu. itatsi might not have accepted the invasion of Mu. sibirica from the continent by competitive exclusion (Hardin, 1960; Cardillo et al., 2008). Although there are some differences in dietary habits and morphological characteristics pertaining to foraging strategy between these species (Yukawa, 1968a; Asahi, 1975; Suzuki et al., 2011), the dietary niche breadth are largely overlapped (Table 1) (Asahi, 1975). Therefore “ecological constraints (e.g., overlaps of their dietary niche)” would not allow two congeneric Mustela species to coexist in the Japanese main islands. It is not obvious whether the effect of inconsistency with the generalist-beforespecialist rule did not allow the migration of Mu. sibirica later than Mu. itatsi, where the former is slightly generalized than the latter. The other explanation for the absence of Mu. sibirica in the Japanese main islands may be associated with a geological constraint. Hosoda et al. (2000) suggested that the Cytb gene haplotype of Mu. sibirica in Tsushima Islands is the same as that observed in Korea. This implies that Korean Strait could have been used as a land bridge recently, while Tsushima Strait was not available. Concordantly, the leopard cat P. bengalensis in Tsushima Islands was shown to be very closely related to the continental Far East lineage, in which the divergence time was estimated to be ca. 0.03 Mya, while this species is not present in the Japanese main islands (Tamada et al., 2008). Furthermore, Mu. sibirica in Jeju Islands is also demonstrated to be not genetically differentiated from that in Korean mainland (Koh et al., 2012). Therefore eastern marginal islands around the Korean Peninsula could have been inhabited by Mu. sibirica in the geological situation that Tsushima Islands were not connected to the Japanese main islands. Thus, the geological constraint might have efficiently worked in the formation of the allopatric distributions of Mu. sibirica and Mu. itatsi, although further improvement of geological knowledge would be needed for Korean and Tsushima Straits.
From the fossil evidence in Honshu Islands, Mu. erminea was found from layers of the Middle and Late Pleistocene periods, while Mu. nivalis was only from Late Pleistocene (Kawamura et al., 1989). Thus, it seems plausible that Mu. erminea was the earlier resident in Honshu than Mu. nivalis. The fact that the distribution of Mu. erminea is more expanded to the southern region than Mu. nivalis (Fig. 2E, F) (Masuda, 2009a, 2009b) may be the consequences of the establishment order. This idea agrees with the generalist-before-specialist hypothesis, because Mu. nivalis is considered more specialized to flesh preys than Mu. erminea (Elmeros, 2006). However, both genetic and morphological data have suggested that Mu. nivalis possesses more geographic variations than Mu. erminea across the Holarctic region (Abramov and Baryshnikov, 2000; Kurose et al., 2005), implying earlier diversifications among Mu. nivalis lineages than Mu. erminea. Therefore, in a future study, it will be necessary to assess more precise demographic history and the chronology of both species in order to evaluate which species migrated into the Japanese archipelagos first. Irrespective of the histories of both species, strict niche partitions might be needed for coexistence of the congeneric species whose niches are similar to each other. Available ecological data of these species in Japan are not sufficient to satisfactorily understand the mechanisms of their coexistence. However, if the ecological features are not extremely varied across the Eurasian continent and Japan, they can inhabit the same places by slightly different niche requirements. Dietary contents differ a bit from each other, as described above, where the niche breadth of Mu. erminea is slightly larger than Mu. nivalis (McDonald et al., 2000; Elmeros, 2006). Since Mu. nivalis is socially subordinate to dominant Mu. erminea (Erlinge and Sandell, 1988), Mu. nivalis would have to utilize resources that do not overlap with Mu. erminea (Morse, 1974). Mu. nivalis can more effectively catch small rodents by using its smaller body size and can breed more rapidly without delayed implantation (King and Moors, 1979). These differences in feeding and breeding strategies would reduce competitions between two species. In other words, “ecological constraints” may force two closely related species to divide their niche for their coexistence. Clearly further studies on phylogeography and dietary habits are needed to elucidate the historical and ecological causes for the assemblage of Mu. erminea and Mu. nivalis in Japan.
Phylogeographic and ecological factors are both important facets in the species assemblage. By answering the four questions posed above, it was inferred that three constraints from evolutionary (or phylogenetic), geological, and ecological perspective have played effective roles in mustelid faunal assemblages in Japan. The large evolutionary difference tends to permit the coexistence without any major problems. Ecological constraints would force two closely related species to be allopatric by competitive exclusion or to be sympatric by resource partitions. Although these evolutionary and ecological constraints would have strong effects on the species assemblages, I suppose that the geological constraints would probably have the priority to generate the co-occurrence of species. If the geological situations do not permit species to be pooled in a particular place, it is obvious that the evolutionary or ecological causes would not work anymore. The mammalian faunal assemblage in the Japanese archipelagos would have been largely constrained by its complicated geohistory such as vicariance in the major straits in different ages. Geological effects would not be avoided in deciding which species is encompassed in the Japanese archipelagos. Further multidisciplinary studies would be needed to untangle the complicated effects on the faunal assemblage process leading to current species distributions.
The phylogeographic and feeding ecological data are still insufficient to fully understand the relative strength of the aforementioned three factors. For further refinements of our knowledge about the importance of evolution (or phylogeny), geology, or ecology in the formation of the mustelid fauna in Japan, at least four issues need to be addressed. First, phylogeographic and demographic history of the resident species in Japan should be clarified with more fine scale time axis. Second, various niche spaces such as habitat preferences should be assessed in addition to dietary habits to examine niche breadth and overlap in more broad ecological sense. Interspecific interactions between introduced and native species in Japan (e.g., Mu. sibirica and Mu. itatsi in the western part of Honshu Islands) may provide valuable insight into the importance of the ecological constraint. Third, niche comparisons to carnivoran species other than mustelids occupying similar trophic positions would also be needed. For example, Me. anakuma in Mt. Nyugasa has a diet that is similar to Nyctereutes procyonoides (the raccoon dog) classified into different family (Canidae) than to Ma. melampus in the same family, possibly causing competitive interactions (Yamamoto, 1994; Koike et al., 2012). Therefore, considering only mustelids is insufficient to discuss the mechanisms of coexistence in the light of the ecological niche overlap and partition. Finally, the phylogenetic history of prey species should also be clarified to discuss the detail of the ecological constraints on the distribution of the Japanese mustelids. Clarifying phylogeography and ecology of an organism would satisfy the demand from the conservation biology that endemism and adaptation should both be considered (Ryder, 1986; de Guia and Saitoh, 2007; Isaac et al., 2007; Allendorf et al., 2010; Collen et al., 2011; Funk et al., 2012). Addressing the issues raised above will lead to appropriate conservation for small carnivoran species in one of the cradles of biodiversity in East Asia.













![A diagram for configurations of islands around the Japanese archipelagos (Sakhalin [Karafuto], Hokkaido, Honshu, Shikoku, Kyushu, Tsushima, Ryukyu, Jeju, and Taiwan Islands) and major straits (Tatar [Mamiya], La Perouse [Soya], Tsugaru, Tsushima, Korean, Tokara, and Kerama Straits).](http://oak.go.kr/repository/journal/12139/DMBRBT_2013_v29n2_99_f001.jpg)