The genus Actinotrichia Decaisne (1842) is characterized from the other genera in the family Galaxauraceae by its isomorphic life history, the presence of the persistent assimilatory filaments on the thallus surface, and the presence of paraphyses and pericarp in the cystocarp (Decaisne 1842, Weber-van Bosse 1921, Tseng 1941, Svedelius 1952, Wang and Chiang 2001, Liu and Wang 2009). Phylogenetic analyses also provided strong clues to recognize Actinotrichia as a distinct genus. (Huisman et al. 2004, Wang et al. 2005, Liu and Wang 2009).
Actinotrichia consists of four species: A. calcea Pham- Hoang Ho, A. fragilis (Forsskal) Børgesen (the generitype), A. robusta Itono, and A. taiwanica Liu et Wang. Actinotrichia calcea is a Vietnamese species that has small and thin fronds (Pham-Hoang 1978). The widespread A. fragilis occurs throughout the Indo-Pacific (Huisman and Womersley 1994, Silva et al. 1996, Yoshida 1998). A. robusta, described from the specimen collected in the Ryukyu Islands, is reported in Japan and Taiwan (Itono 1979, Liu and Wang 2009), and A. taiwanica was recently described in Taiwan (Liu and Wang 2009). According to literature, both A. fragilis and A. robusta were reported in Japan and Taiwan, but only A. fragilis has been documented in many countries, including Korea (Lee and Kang 2001, Lee 2008, Hwang and Kim 2011, Boo and Ko 2012) and Thailand (Lewmanomont and Ogawa 1995, Lewmanomont et al. 1995).
Galaxauracean red algae have been recognized with molecular tools such as plastid rbcL and nuclear ribosomal cistron regions (Huisman et al. 2004, Kurihara et al.2005, Wang et al. 2005, Liu and Wang 2009). Recently, mitochondrial cox1 gene has been used for identification of red algae (Saunders 2005, Geraldino et al. 2006, Sherwood et al. 2010), and only two cox1 sequences of A. fragilis are to date registered in GenBank. Together with morphological observations, we employed both rbcL and cox1 gene sequencing so as to provide a better understanding of genetic and species diversity in Actinotrichia.
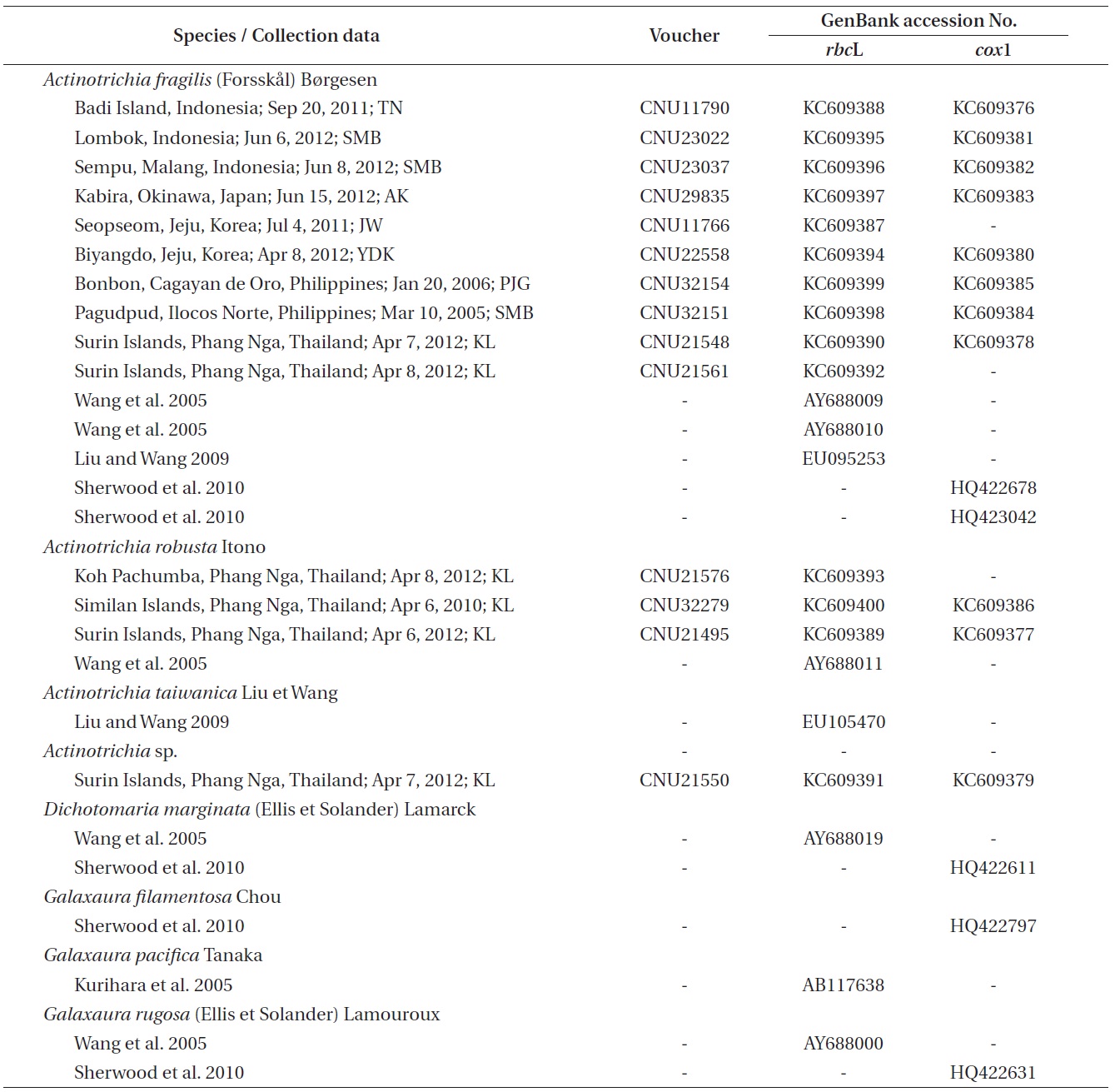
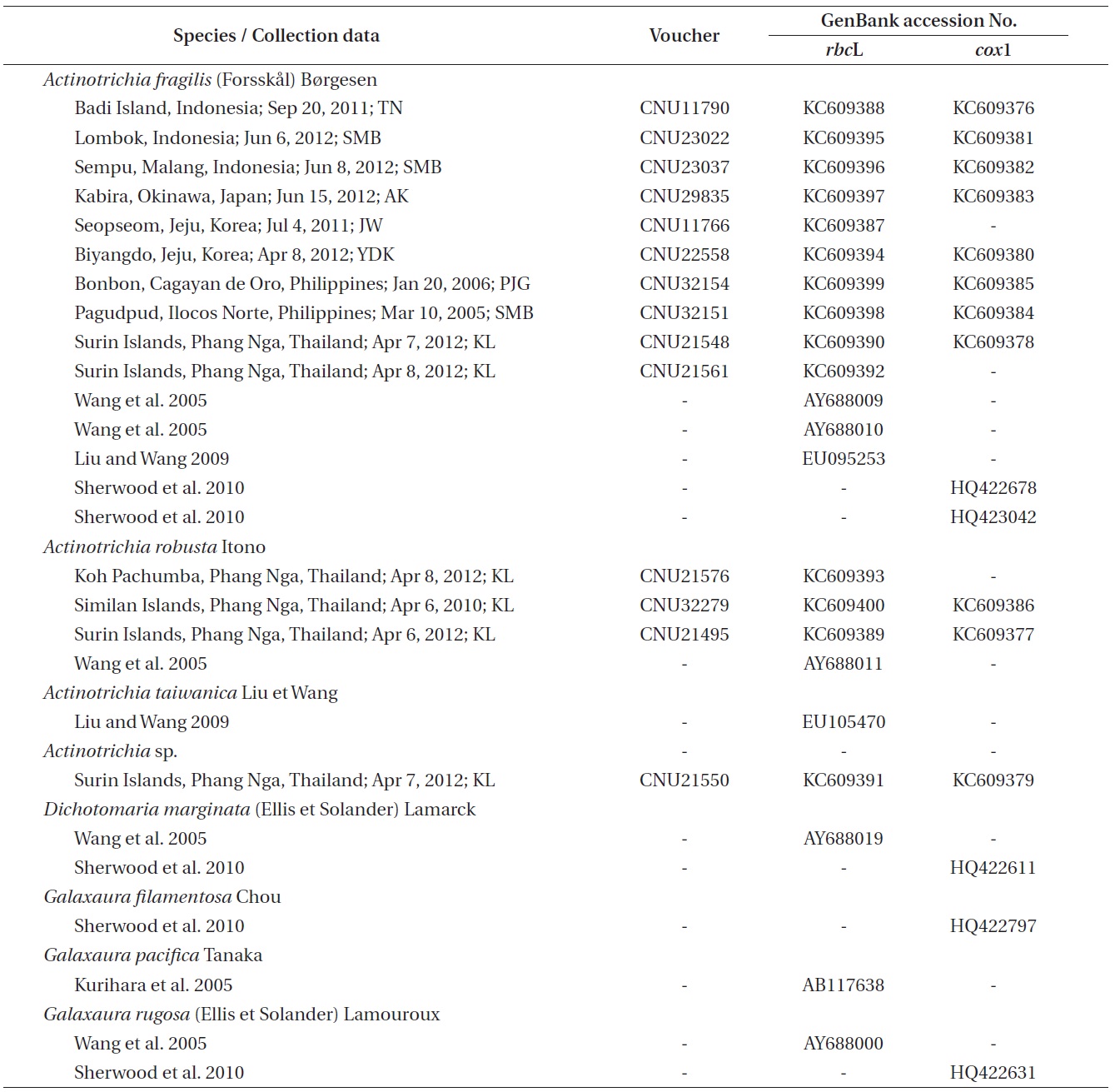
Individuals were collected by snorkeling or scuba diving throughout the western Pacific and the Indian Oceans (Table 1). Specimens for morphological observations were first pressed onto herbarium sheets. Subsequently, distal branch portions were decalcified in 1% HCl solution and sectioned by hand using a razor blade. The sectioned preparations were stained with 1% aqueous aniline blue. Photographs were taken with an FX-35DX camera (Nikon, Tokyo, Japan) attached to a Vanox AHBT3 microscope (Olympus, Tokyo, Japan). Voucher specimens are housed at the herbarium of Chungnam National University, Daejeon, Korea (CNUK).
Tissue from these individuals used for morphological observations and others were desiccated in silica gel. DNA extraction, PCR amplification, and sequencing are described in Geraldino et al. (2010). Specific primer pairs for the amplification and sequencing of each gene were: 1) rbcL, F7-R753 and F645-RrbcS start (Freshwater and Rueness 1994, Lin et al. 2001, Gavio and Fredericq 2002) and 2) cox1, cox143F-C880R (Geraldino et al. 2006, Yang et al. 2008).
Twenty-two rbcL sequences, including 8 accessions from GenBank, and 16 cox1 sequences, including 5 accessions from GenBank, of Actinotrichia and three outgroups (two Galaxaura and one Dichotomaria species) were collated using the multiple-sequence editing program Se-Al v.2.0a11 (Rambaut 1996) and aligned visually. Maximumlikelihood (ML) phylogenetic analyses were performed using RAxML (Stamatakis 2006) using the GTR + Γ model of evolution. We used 200 independent tree inferences with the “number of run” option set to default optimized subtree pruning and regrafting (SPR) rearrangement and 25 distinct rate categories to identify the best tree. Statistical support for each branch was obtained from 1,000 bootstrap replications using the same substitution model and RAxML program settings.
Bayesian analyses of the sequence alignment for
rbcL and cox1 sequences (MrBayes v3.1) (Ronquist and Huelsenbeck 2003) used the Metropolis-coupled Markov chain Monte Carlo method (MC3) with the GTR + Γ + I model and 2 million generations in two independent runs performed with four chains, respectively, and with trees sampled every 100th generation. The 2,800 burn-in period for rbcL and 2,400 burn-in period for cox1 were identified graphically by tracking likelihoods at each generation to determine whether the likelihood values had reached a plateau. The 17,201 trees for rbcL and 17,601 trees for cox1 sampled at stationarity were used to infer the Bayesian posterior probability. Majority-rule consensus trees were calculated using PAUP* 4.0b10 (Swofford 2002).
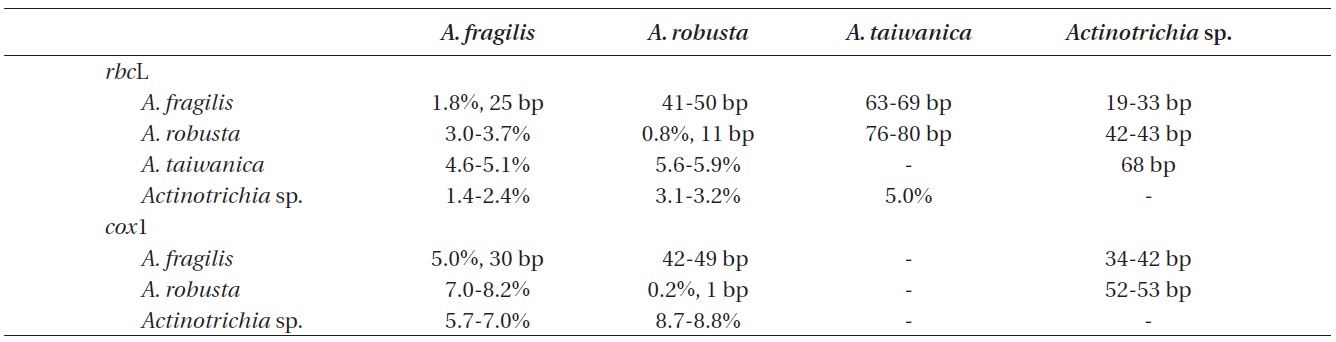
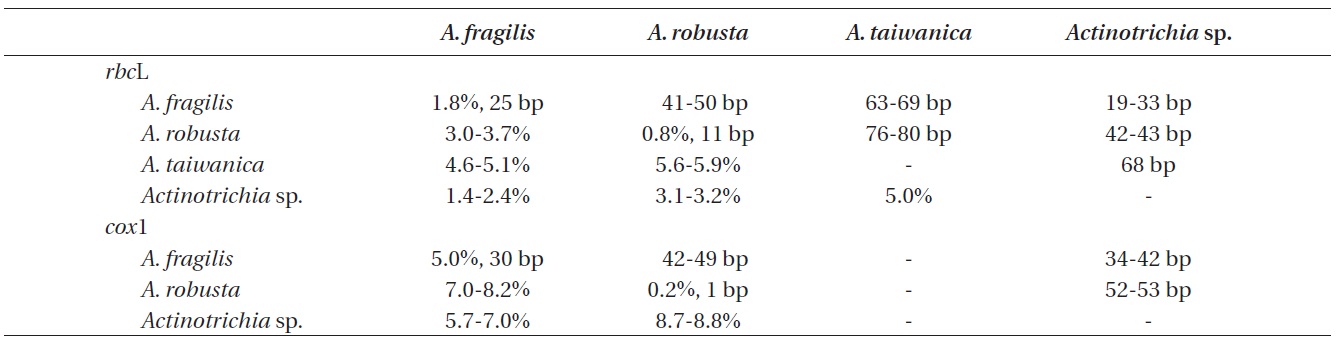
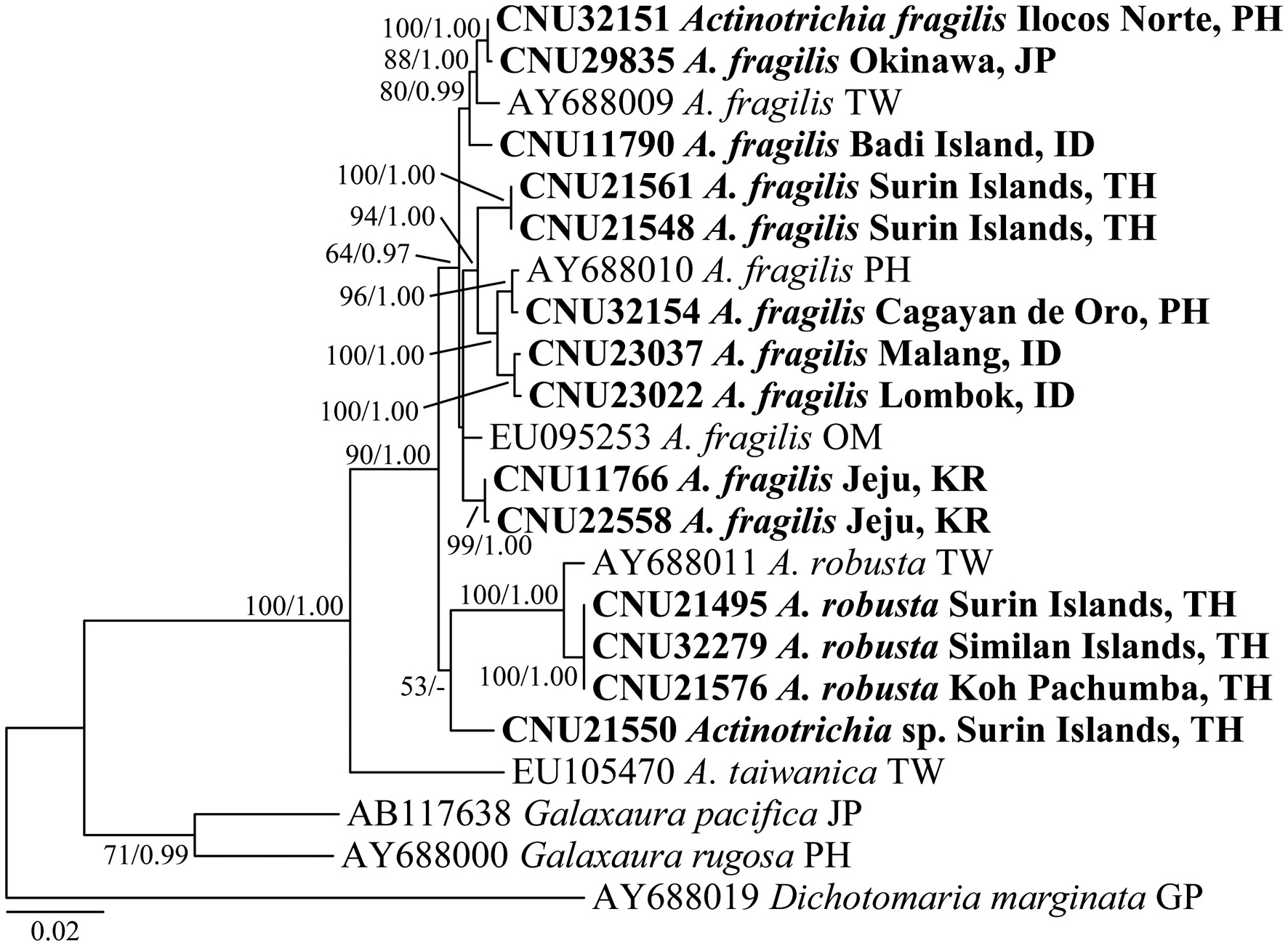
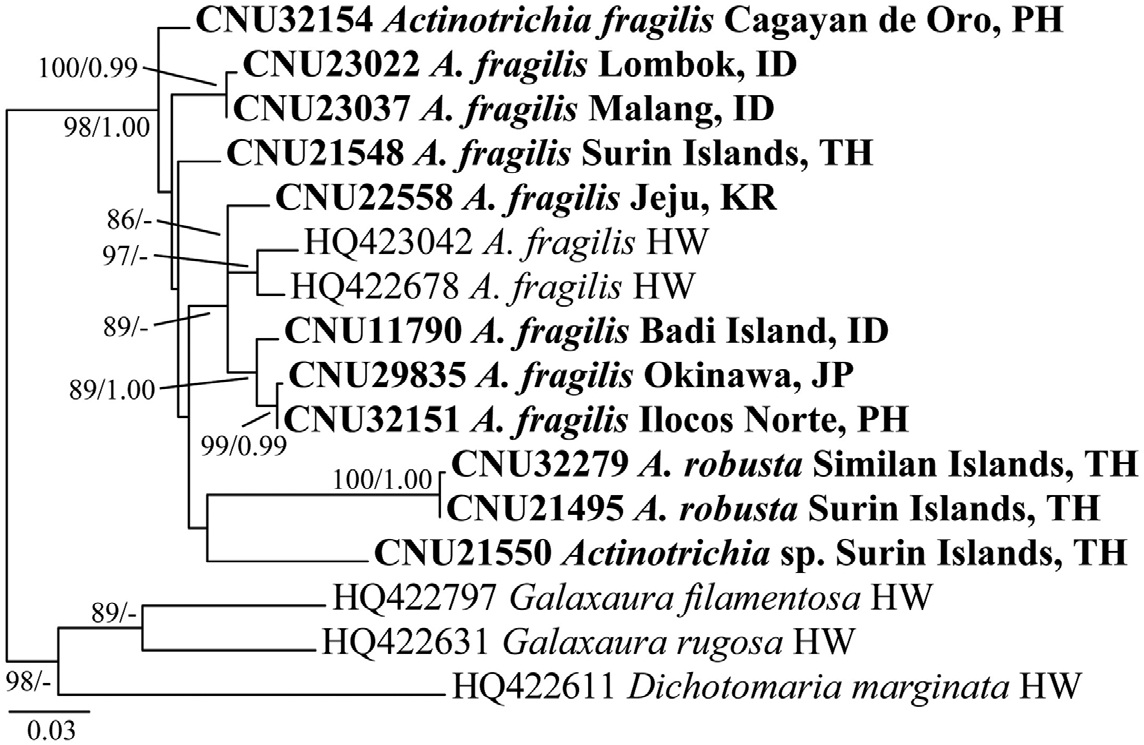
A 1,382 bp region of rbcL for 22 individuals were aligned, consisting of A. fragilis (13), A. robusta (4), A. taiwanica (1), Actinotrichia sp. (1), and outgroups (3). Variable sites occurred at 280 positions (20.3%), and 152 positions (11.0%) were parsimoniously informative. Intraspecific pairwise divergence was 1.8% (25 bp) in A. fragilis and 0.8% (11 bp) in A. robusta. Interspecific pairwise divergence was 41-50 bp (3.0-3.7%) between A. fragilis and A. robusta, and 76-80 bp (5.6-5.9%) between A. robusta and A. taiwanica. Actinotrichia sp. differed by 19-33 bp (1.4-2.4%) from A. fragilis, 42-43 bp (3.1-3.2%) from A. robusta, and 68 bp (5.0%) from A. taiwanica (Table 2). Our rbcL tree revealed that all four species were clearly separated but collectively they formed a monophyletic clade. A. taiwanica formed the sister relationship to the clade of the remaining three taxa. Relationships among A. fragilis,
A. robusta, and Actinotrichia sp. were not resolved (Fig. 1).
A 601-nucleotide portion of the cox1 gene was aligned for 16 specimens: A. fragilis (10), A. robusta (2), Actinotrichia sp. (1), and outgroups (3). Variable sites occurred at 149 positions (24.8%), and 95 positions (15.8%) were parsimoniously informative. Intraspecific pairwise divergence was up to 5.0% (30 bp) in A. fragilis, and sequences of A. robusta from three different islands in Thailand were identical. A. robusta and A. fragilis differed by 42-49 bp (7.0-8.2%). Actinotrichia sp. differed by 34-42 bp (5.7- 7.0%) from A. fragilis and 52-53 bp (8.7-8.8%) from A. robusta. The cox1 tree (Fig. 2) revealed that all three taxa were clearly distinct.
A. fragilis (Fig. 3A) was attached to hard substrata in the intertidal and subtidal zones in warm and temperate waters
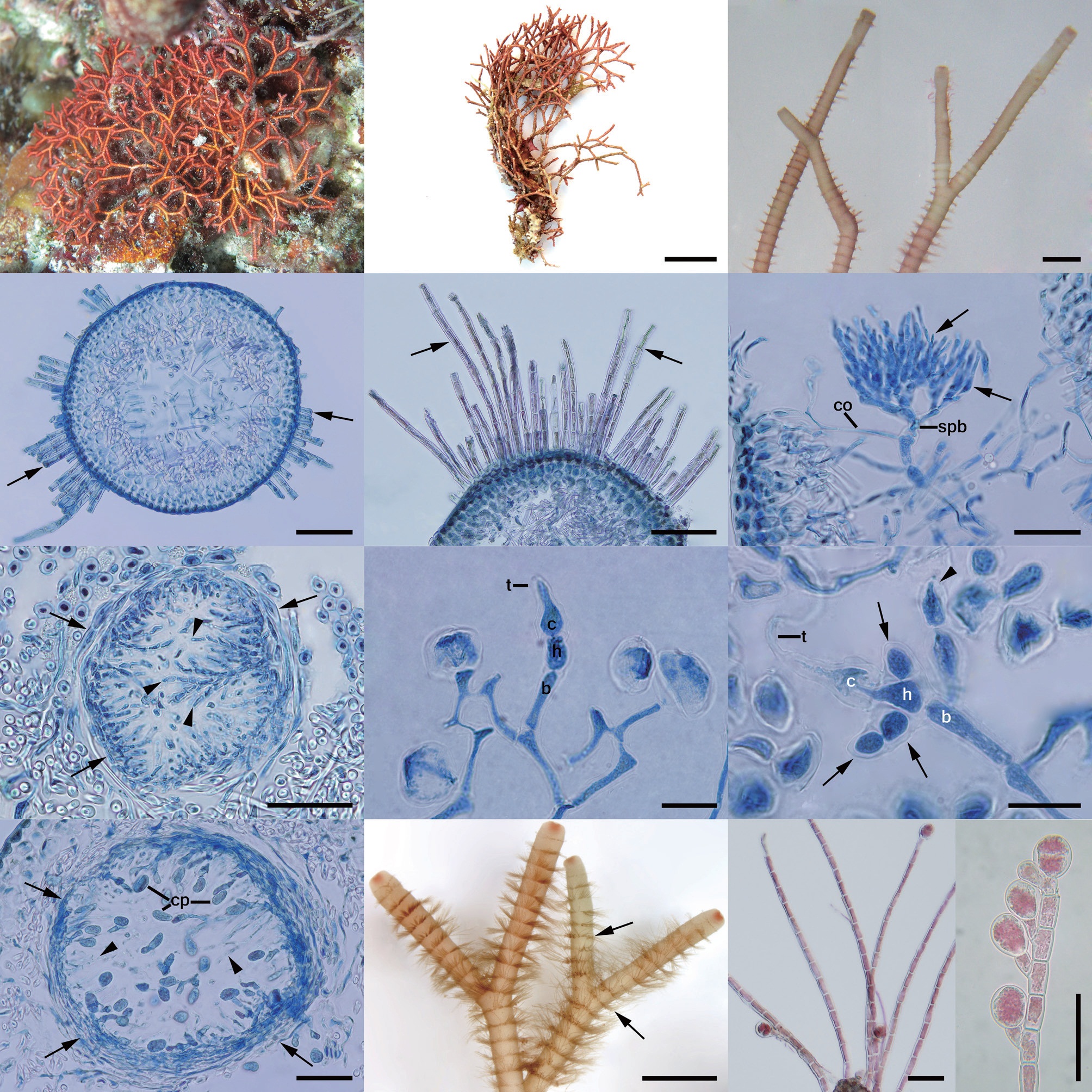
[Fig. 3.] Actinotrichia fragilis (Forsskal) Børgesen. (A) Morphology of thallus in Cebu, Philippines. (B) Morphology of herbarium specimen collected in Ilocos Norte, Philippines. (C) Dichotomously branched thallus. (D) Section of gametophyte branch with assimilatory filaments arising from the outermost cortical cells (arrows). (E) Three to four cortical cell layers with assimilatory filament (arrow) and medullar portion. (F) Young spermatangial branch with primary spermatangial filament (arrowhead) and secondary spermatangial filaments (arrows). (G) Spermatangial conceptacle showing the numerous terminal spermatangia (arrowheads) and peripheral layer of conceptacle (arrows). (H) Early carpogonial branch showing carpogonium with trichogyne, hypogynous with 2-sterile branches (arrows) and basal cell. (I) Developed carpogonial branch showing carpogonium with trichogyne, hypogynous with enlarge sterile branch (arrowheads) and basal cell bearing numerous involucral filaments (arrows). (J) Mature cystocarp showing gonimoblast filament bearing carposporangia and paraphyses (arrowheads) arising from pericarp (arrows). (K) Tetrasporophyte branches with assimilatory filaments (arrows). (L) Terminal and lateral tetrasporangia on assimilatory filaments. (M) Cruciate tetrasporangia. b, basal cell; c, carpogonium; cp, carpospores; g, gonimoblast; h, hypogynous cell; spb, spermatangial branch; t, trichogyne. Scale bars represent: B, 1 cm; C & K, 1 mm; D, 100 μm; E, G, J, L & M, 50 μm; F, H & I, 20 μm.
[Fig. 4.] Actinotrichia robusta Itono. (A) Morphology of thallus in Similan Islands, Thailand. (B) Morphology of herbarium specimen collected in Surin Islands, Thailand. (C) Branches appearing alternate. (D) Section of gametophyte branch with assimilatory filaments arising from the outermost cortical cells (arrows). (E) Three to four cortical cell layers with assimilatory filaments (arrows) and medullar portion. (F) Young spermatangial branch with several secondary spermatangial filaments (arrows). (G) Spermatangial conceptacle showing numerous terminal spermatangia (arrowheads) enclosed within peripheral layer of conceptacle (arrows). (H) Carpogonial branch initial showing carpogonium with trichogyne, hypogynous, and basal cell. (I) Young carpogonial branch showing carpogonium, hypogynous bearing lateral sterile branches (arrows), and basal cell with involucral filament initial (arrowhead). (J) Mature cystocarp with carposporangia arising along inner surface of pericarp (arrows) and paraphyses (arrowheads) intermixing with the gonimoblast filaments. (K) Tetrasporophyte branches with assimilatory filaments (arrows). (L) Terminally and laterally tetrasporangia on assimilatory filaments. (M) Well developed tetrasporangia. b, basal cell; c, carpogonium; co, cortex; cp, carpospores; h, hypogynous cell; spb, spermatangial branch; t, trichogyne. Scale bars represent: B, 1 cm; C & K, 1 mm; D & E, 100 μm; F, G, J, L & M, 50 μm; H & I, 20 μm.
in southeast and northeast Asian waters.
The orange to red thalli were calcified, stiff, and erect but somewhat sprawling, up to 8 cm in height (Fig. 3B), arising from a discoid holdfast (3-5 mm diam.). Dichotomous branches (400-600 μm diam.) occurred at intervals of 3-6 mm and angles of 40-60°, with distinct whorled assimilatory filaments (Fig. 3C). The cortex consisted of three to four layers with an innermost layer of cuboidal cells (18-22 × 13-18 μm), grading to outermost cells of spherical cells (10-15 μm diam.). Five- to twelve-celled assimilatory filaments arose from some portions of epidermal cell and formed successive whorls on the thallus surface (Fig. 3D & E).
Gametophytes were isomorphic to tetrasporophytes, and gametophytes were dioecious. Spermatangial branch initials arose from an ordinary filament near the apex of branches (Fig. 3F) and they divided laterally and transversely to form a hemispherical conceptacle (200-250 μm diam.). The inner cortical cells of the conceptacles produced numerous secondary spermatangial filaments that cut off ovoid spermatangia (5-8 × 3-6 μm) (Fig. 3G).
Cystocarps were commonly found in the distal parts of the thallus. Carpogonial branch initials were three-celled, consisting of carpogonium with elongated trichogyne, hypogynous cell, and basal cell (Fig. 3H). Then, hypogenous cell cut off one to two sterile branches and the basal cells produced four to five involucral filaments that ultimately formed the pericarp (Fig. 3I). Cystocarps were hemispherical and 250-300 μm diam. Ovoid carposporangia were 18-30 × 10-15 μm in size, and were produced singly and terminally from the gonimoblast filaments, which restricted only at the basal portion of the cystocarp. Three to six-celled sterile paraphyses arose from the pericarp projected into the cystocarp cavity and were not mixed with gonimoblast filaments (Fig. 3J).
Tetrasporangia (Fig. 3K) were formed terminally or lat-erally on assimilatory filaments of tetrasporophytes. Tetrasporangia were cruciately divided, spherical to ovoid in shape, and 15-25 μm diam. (Fig. 3L & M).
A. robusta (Fig. 4A) was usually attached to rocky reefs, rocks and other hard substrata in the subtidal zone of three isolated islands in the Andaman Sea. Thalli were collected mostly in March and April. Seawater temperatures in April in Thailand ranged from 26 to 30℃. The orange to red thalli were heavily calcified and erect to sprawled (Fig. 4A), up to 8 cm in height (Fig. 4B), arising from the discoid holdfast (3-5 mm diam.). Branches were repeatedly dichotomously divided and intervals of branching were 3-6 mm in length and 400-600 μm diam., branching angles between 30-70°, with indistinct whorled assimilatory filaments (Fig. 4C). Thalli were multiaxial, consisted of three to four cortical cell layers and filamentous medulla. The innermost cortical cells were obovoid (18-22 × 13-18 μm), grading to pigmented outermost cortical cells of 10- 15 μm diam. Five- to twenty-celled assimilatory filaments arose from some portions of epidermal cell and formed indistinct whorls on the thallus surface (Fig. 4D & E).
Gametophytes were dioecious. Spermatangial branch initials formed in place of ordinary filaments near the apex of branches (Fig. 4F), then they grew distally and laterally to form a hemispherical conceptacle (200-250 μm diam.) (Fig. 4G). The inner cells of the conceptacles produced numerous secondary spermatangial filaments that cut off obovoid spermatangia (2-6 μm diam.). The structure and development of carpogonial branches mostly agreed with the genetic type (Fig. 4H & I). Cystocarps were hemispherical, 200-350 μm diam. Ovoid to obovoid carposporangia (15-30 × 8-15 μm) were produced singly and terminally from the gonimoblast filaments, which distributed along the inner surface of the cystocarp. Threeto six-celled sterile paraphyses arising from the pericarp projected into the cystocarp cavity and intermixed with gonimoblast filaments (Fig. 4J).
Tetrasporangia were formed terminally or laterally on assimilatory filaments of tetrasporophytic thalli (Fig. 4K). Mature tetrasporangia were cruciately divided, spherical to ovoid in shape, and 15-25 μm diam. (Fig. 4L & M).
Actinotrichia sp. was attached on corals in sandy bottom of the subtidal zone in Surin Islands, Thailand. Thalli were cream to pink in color, calcified, terete, and approximately 2.5 cm in height (Fig. 5A). Branches were dichotomously divided, and intervals of branches were short with less than 1 cm (Fig. 5B). The cortex consisted of three layers with an innermost layer of obovoid cells (18-21 × 14-17 μm), grading to outermost cells of spherical cells (10-15 μm diam.). Five- to sixteen-celled assimilatory filaments arose from some portions of epidermal cell and formed successive whorls on the thallus surface (Fig. 5C).
Our analyses of rbcL and cox1 genes confirmed the presence of three species of Actinotrichia in the western Pacific and the Indian Oceans: A. fragilis, A. robusta, and Actinotrichia sp. The distribution of A. fragilis is widespread, occurring in Indonesia, Japan, Korea, Philippines, and Thailand (present study), as well as Oman, Philippines, and Taiwan (Wang et al. 2005, Liu and Wang 2009). Morphological differences were apparent, as A. fragilis from Philippines and Thailand was erect to sprawled in axes, while those from Indonesia, Japan, and Korea were erect only. A. fragilis is distinguished by wide angle of branches with more than 40°, gonimoblasts compacted in the center of the cystocarp, and paraphyses not intermixed with gonimoblasts (Liu and Wang 2009). Using only the DNA barcoding cox1 gene, intraspecific pairwise divergence was very high (5.0% or 30 bp difference) within A. fragilis compared to other red algae in which intraspecific differences ranged from 0.3% (2 bp) (Saunders 2005) to 0.9% (11 bp) (Yang et al. 2008). The high genetic diversity of A. fragilis shown in the present study may be due to inclusion of multiple specimens from widely separated countries (e.g., Oman to Japan). Alternately, A. fragilis may be a species complex containing several cryptic species, as recently shown in other marine algae (Kucera and Saunders 2012, Lee et al. 2013). Analysis of specimens from Australia, Egypt, Hawaii, Kenya, Pacific Island, and South Africa (see Guiry and Guiry 2013) is necessary to further understand intraspecific divergence in A. fragilis.
Our A. robusta collections from Thailand correspond in their habits and in the structures of the cortex, medulla, and reproductive structures to the description of the species from Japan (Itono 1979) and Taiwan (Liu and Wang 2009). A. robusta differs from A. fragilis and A. taiwanica in morphology (Figs 3 & 4) and in interspecific divergences in rbcL and cox1 sequences (Table 2). Morphologically, A. robusta is characterized by regularly dichotomous branches with indistinct whorled assimilatory filaments, a various branching angle (30-70°), and paraphyses issuing from the pericarp, which intermix with gonimoblasts. This is the first report of A. robusta in the Andaman Sea, and represents a significant extension from the East China Sea where the species is common (Itono 1979, Liu and Wang 2009). It is likely that A. robusta also extends to other southeast Asian and Indian Ocean regions where A. fragilis is present (Tsutsui et al. 2005, Pham et al. 2011, Atmadja and Prud’homme van Reine 2012), because it is possible that A. fragilis in these areas includes specimens that would now be identified as A. robusta. Detailed observations of field-collected material will provide a more realistic evaluation of the distribution of A. robusta in the tropical and subtropical waters of the world where A. fragilis occurs.
Actinotrichia sp. from Surin Islands differed from the other species of the genus in both rbcL and cox1 sequences. Dry thalli of this species tend to be more fragile than the relatively tenacious A. fragilis thalli. Our specimens were very similar to the description and photo of A. fragilis from Thailand (Lewmanomont and Ogawa 1995, p. 91) and to A. calcea from Vietnam (Pham-Hoang 1978). Con Dao Island, the southern tip of Vietnam, the type locality of A. calcea may also be relate to the similarity in water temperatures and other environmental conditions to Surin Islands in the Andaman Sea, where our specimens were collected. However, because the type specimen of A. calcea was not available in the present study and because its protologue has limited description and illustrations (Pham Hoang 1978), we save the identification of the Surin Islands specimens until the samples of A. calcea from the type locality will be compared with our specimens.
Abbott (1999) was one of the first researchers to notice the effect of ocean warming on calcified algae with a note that A. fragilis was more common along eastern O’ahu shores formerly (50 years ago) than in 1998. Ocean warming may amplify the negative effects of carbon dioxide on Systemthe health of Actinotrichia thalli, as shown in coralline algae (Martin and Gattuso 2009). Our morphological and molecular study on calcified Actinotrichia will provide a further estimate of the effects of ocean warming on the change of species diversity and distribution of calcified algae.