The Orders Corallinales and Sporolithales represent an evolutionary clade of red algae (Rhodophyta) that are characterized by the deposition of calcite in cell walls (e.g., Johansen 1981). Due to the natural calcification of the members of this clade, they are common in the fossil record (e.g., Aguirre et al. 2010). Species of coralline red algae are widely distributed around the world from the poles to the tropics and from intertidal areas to deep in the oceans (e.g., Steneck 1986). Because of their wide range in ecological and geographical distribution, this clade has several growth forms (e.g., Woelkerling et al. 1993), from endoparasites to free living forms (e.g., Johansen 1981), making the taxonomic delimitation in this clade complex.
Twelve genera and 42 species of coralline red algae have been identified in coastal and offshore areas of the continental shelf of Brazil (reviewed by Creed et al. 2010). From this list of taxa 10 genera and 14 species are cited for the Brazilian reefs. However, many of these species are poorly known and recent taxonomic analyses have
shown that Brazilian coralline flora is more diverse than was previously considered (e.g., Villas-Boas et al. 2009, Da Nobrega Farias et al. 2010, Bahia et al. 2011, Horta et al. 2011, Henriques et al. 2012). Furthermore, most ecological studies were not carried out using rigorous taxonomic evaluation. During the course of ecological studies at Parque Municipal Marinho do Recife de Fora (e.g., Mariath et al. in press) two species of nongeniculate coralline red algae were found as the major species cover on the reef. When vegetative and reproductive anatomies of the species were analyzed we found a new species of the genus
The work was carried out within the limits of the Municipal Marine Park of Recife de Fora in Porto Seguro, southeastern Brazil, which is the first Conservation Unit in this area, established in 1997. The protected area is of approximately 17.5 km² (Fig. 1). The depth surrounding this reef varies from 6 to 8 m near shore, and may reach up to 15 m in the northeast offshore area (e.g., Costa et al. 2002). The Ponto Oeste site (16º24′36″ S, 038º59′08″ W) was visited more than 6 times between Feb 27, 2007 and Jan 28, 2008.
This site located in a portion of the reef flat ranging from 0.5 and 3 m depth and separated from most reefs flat by crevices, reaching 6 m at the reef base. This area inconstantly submerged and distant from reef edge therefore more protected compared to reef slope which is directly exposed to waves. Here the coral colonies are larger than on the reef flat near the reef edge, with coralline algae growing under the sediment, and among and on top of some of the coral colonies.
All material was collected by SCUBA or snorkel diving and maintained in tanks with sea water in a flow through system. Fragments with reproductive structures were fixed in 4% formalin in seawater. For light microscopy, formalin preserved specimens were first decalcified in 10% nitric acid, dehydrated in 30, 60, and 96% alcohol. Three to ten μm sections were cut with a microtome Shandon Hypercut. Individual sections were removed and stained with 1% toluidine blue as described by (e.g., Moura et al. 1997 modified where the alcoholic series was reduced to concentrations of 30, 60, and 95%).
For scanning electron microscopy we follow the methods proposed by Garbary (1978) and Garbary and Johansen (1982) which consist in the use of air-dried material was fractured using finger nails, forceps, diagonal cutters, or a small hammer and cold chisel. The fractured pieces were mounted on stubs, using adhesive tabs double sided carbon tape and colloidal silver liquid, stored in a desiccators for at least 24 h prior to examination, coated with gold for 4-6 min in an Emitech K550X (Quorum Technologies Ltd., Kent, UK), and examined with a Zeiss EVO 40 scanning electron microscope (Carl Zeiss, Oberkochen, Germany) with an accelerating voltage of 15.34 kV. Description of the new species is following the new botanical code of nomenclature (McNeil et al. 2006) in where latin descriptions are no longer require for new species.
Growth form and terminology follows the concepts explained in Woelkerling et al. (1993) along with their related references. All the material used in the present study are deposit in the Herbaria of Rio de Janeiro Botanical Garden (RB according to Holmgren et al. 1990).
Family Hapalidiaceae Subfamily Melobesioideae
>
Lithothamnion steneckii Mariath and Figueiredo (
Holotype specimen. Recife de Fora, Bahia, Feb 27, 2007 (RB 475146, Feb 27, 2007), Rodrigo Mariath and Marcia Figueiredo collectors.
Isotype specimens. Recife de Fora, Bahia, Feb 27, 2007 (RB 475147, RB 475148, RB 517938, RB 517939), Rodrigo Mariath and Marcia Figueiredo collectors.
Type locality. The Municipal Marine Park of the Recife de Fora, situated in the municipal district of Porto Seguro, Bahia.
Etymology. The name of this species is to honor Dr. Robert Steneck’s work on coralline red algae and his particular interest in Brazilian coral reefs.
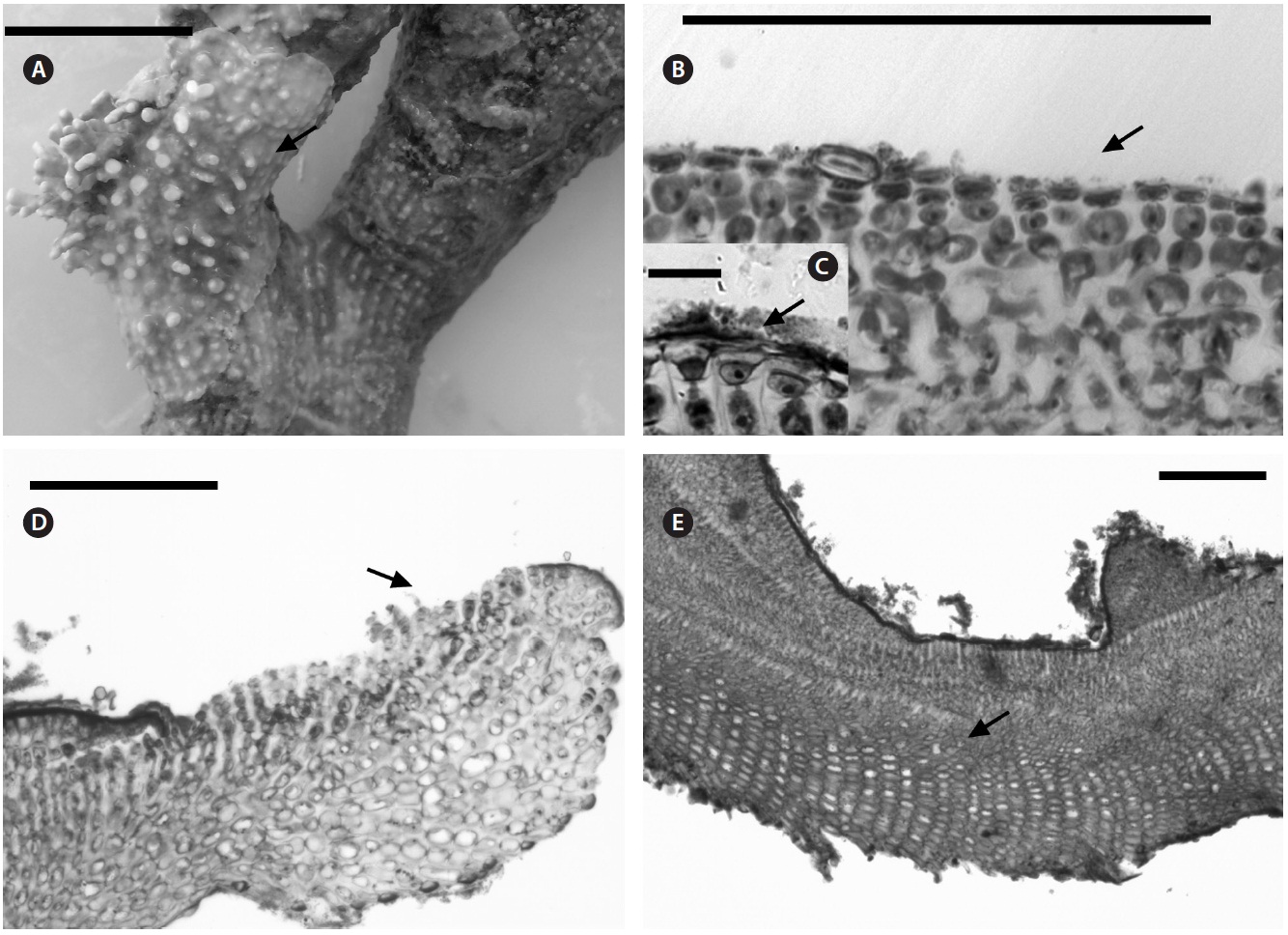
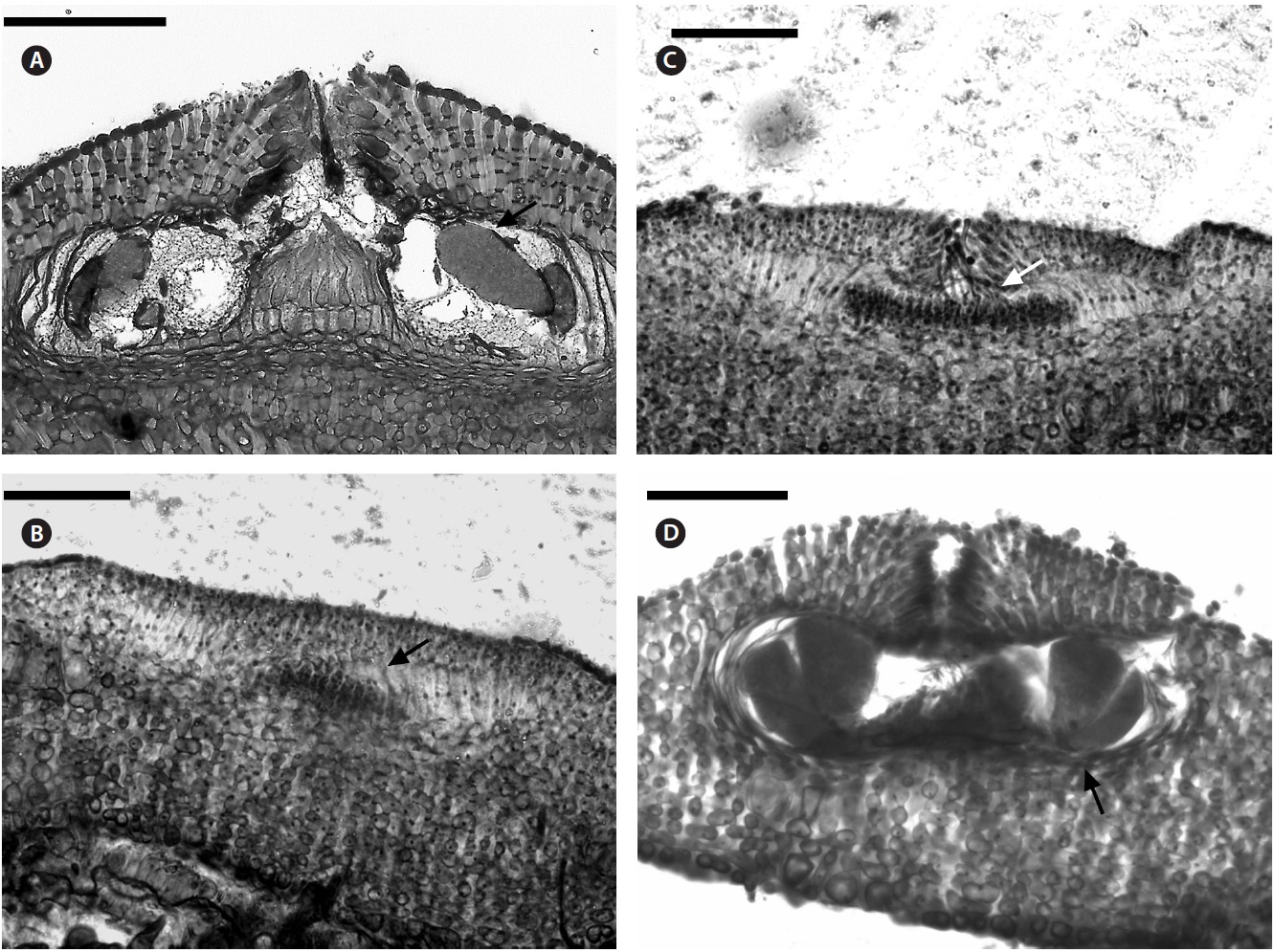
Description. Plants non-geniculate with an encrusting to fruticose growth form (Fig. 2A); thalli thickness 200- 315 μm. Epithelial flared cells 4-5 μm long and 6-8 μm in diameter (Fig. 2B & C) and monomerous construction in a system of a single group of filaments with cell fusions (Fig. 2D) that are parallel to the substrate and giving rise to groups of vertical cells (Fig. 2E). Cells in the central area
are 5-10 μm long and 5-20 μm in diameter and perithallial cells measuring 5-10 μm long and 3-7 μm in diameter.
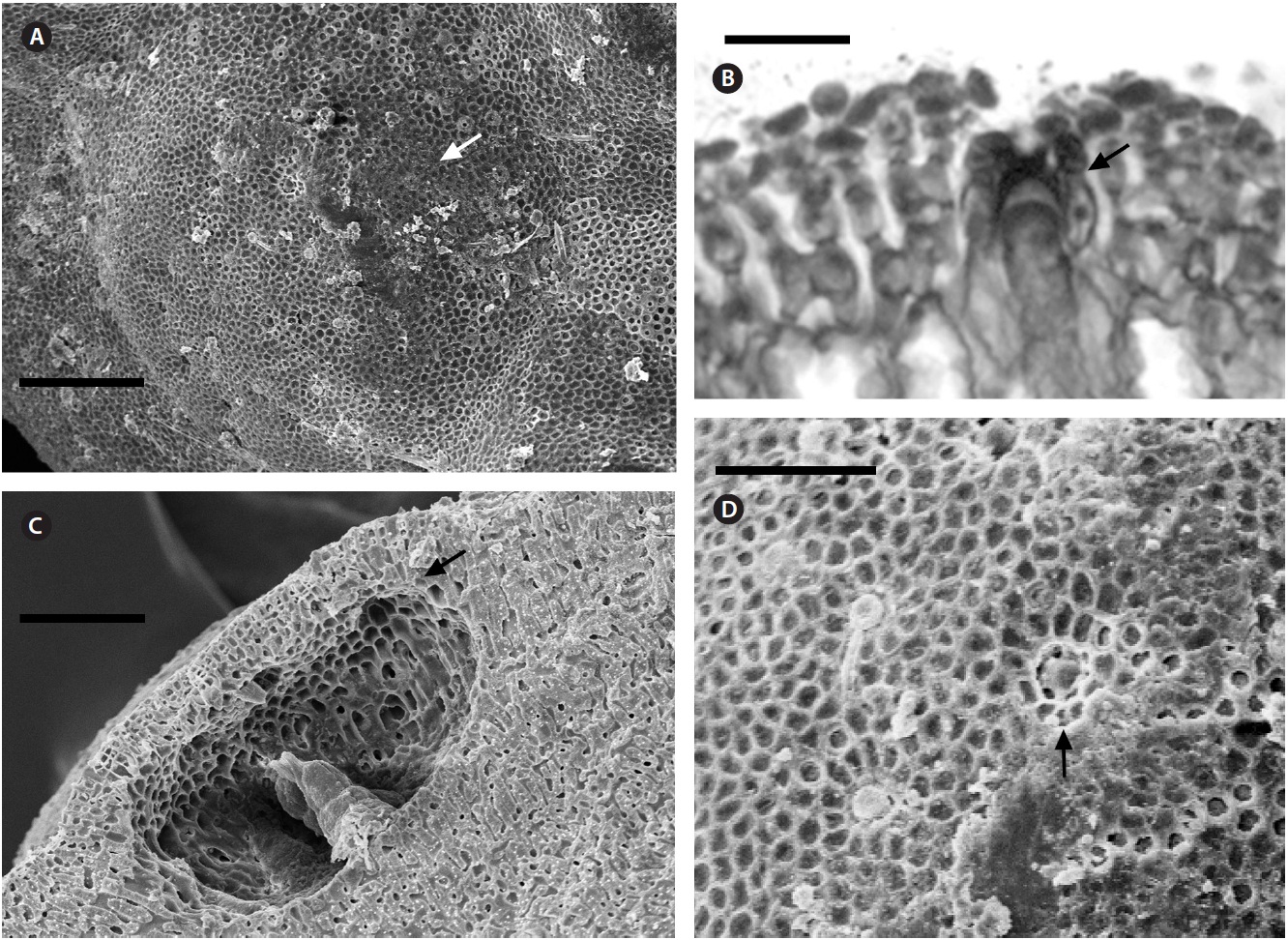
Multiporate tetrasporangial conceptacles (Fig. 3A), high and rounded, with external diameter size measuring 300-335 μm (Fig. 3B & C), rosettes in surface view formed by six or seven cells that lie around the pores (Fig. 3D). The chambers of conceptacles measure 90-200 μm long and 195-330 μm in diameter, showing no collumela with 4-6 cell layers above them and with the presence of apical plugs in pores with 2 cell layers lining the pore (Fig. 3B).
Ecological distribution.
Family Corallinaceae Subfamily Mastophoroideae
>
Pneophyllum conicum (Dawson) Keats, Chamberlain and Baba (
Basionym.
Holotype specimen. (E. Y. Dawson, Nov 19, 1953, Dawson 12148). This material was listed by Dawson (1960) as being in the herbarium of the Baudette Foundation, with an isotype in the herbarium of the A. Hancock Foundation but all the material are now housed in the Herbaria of UC Berkeley.
Type locality. Intertidal reef at Binners Cove, Isla Socorro, Revillagigedo Archipelago, Mexico.
Specimens examined. Recife de Fora, Bahia, Feb 27, 2007 (RB 475144, RB 517933, RB 517934, RB 517935, RB 517936).
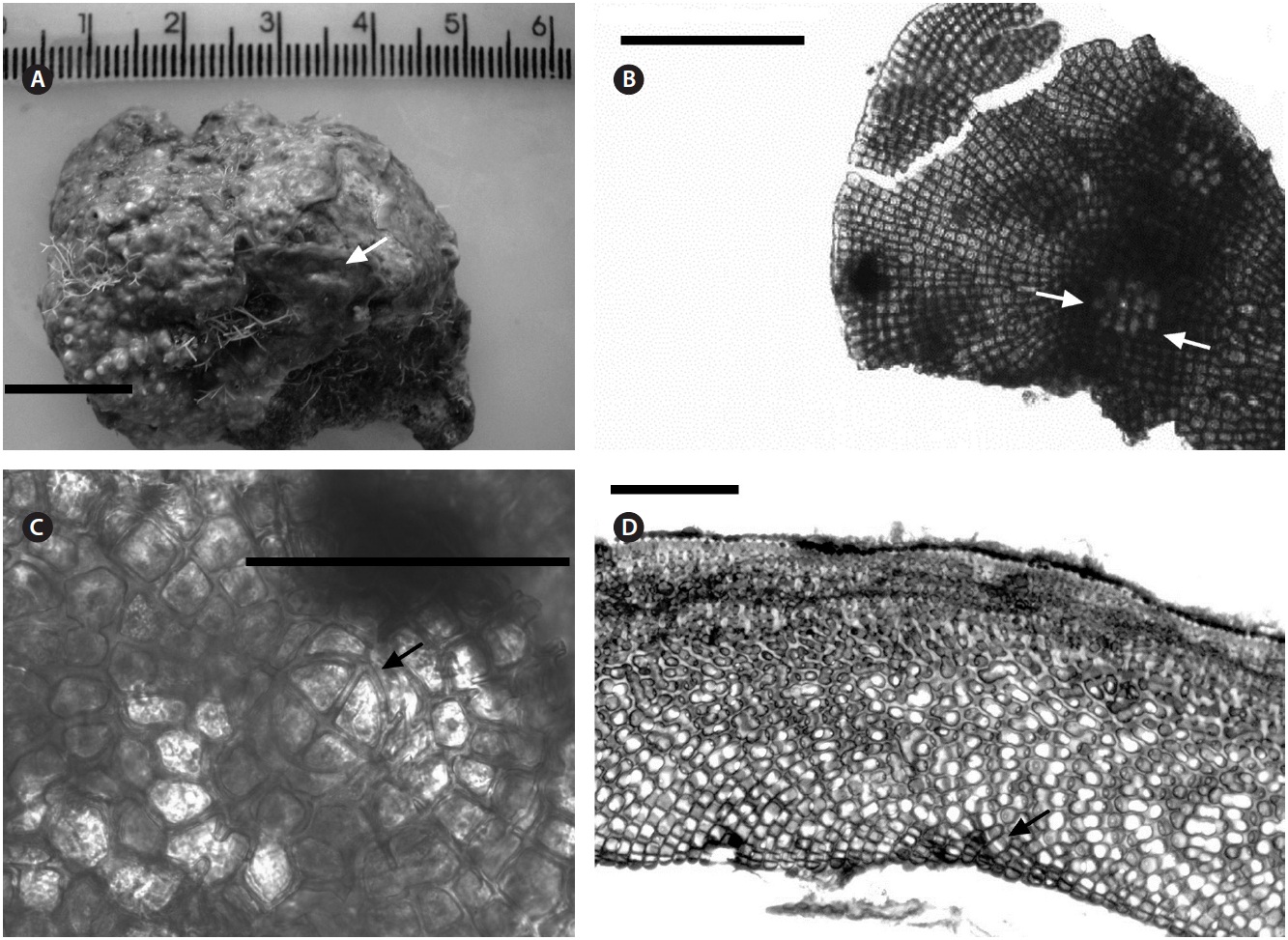
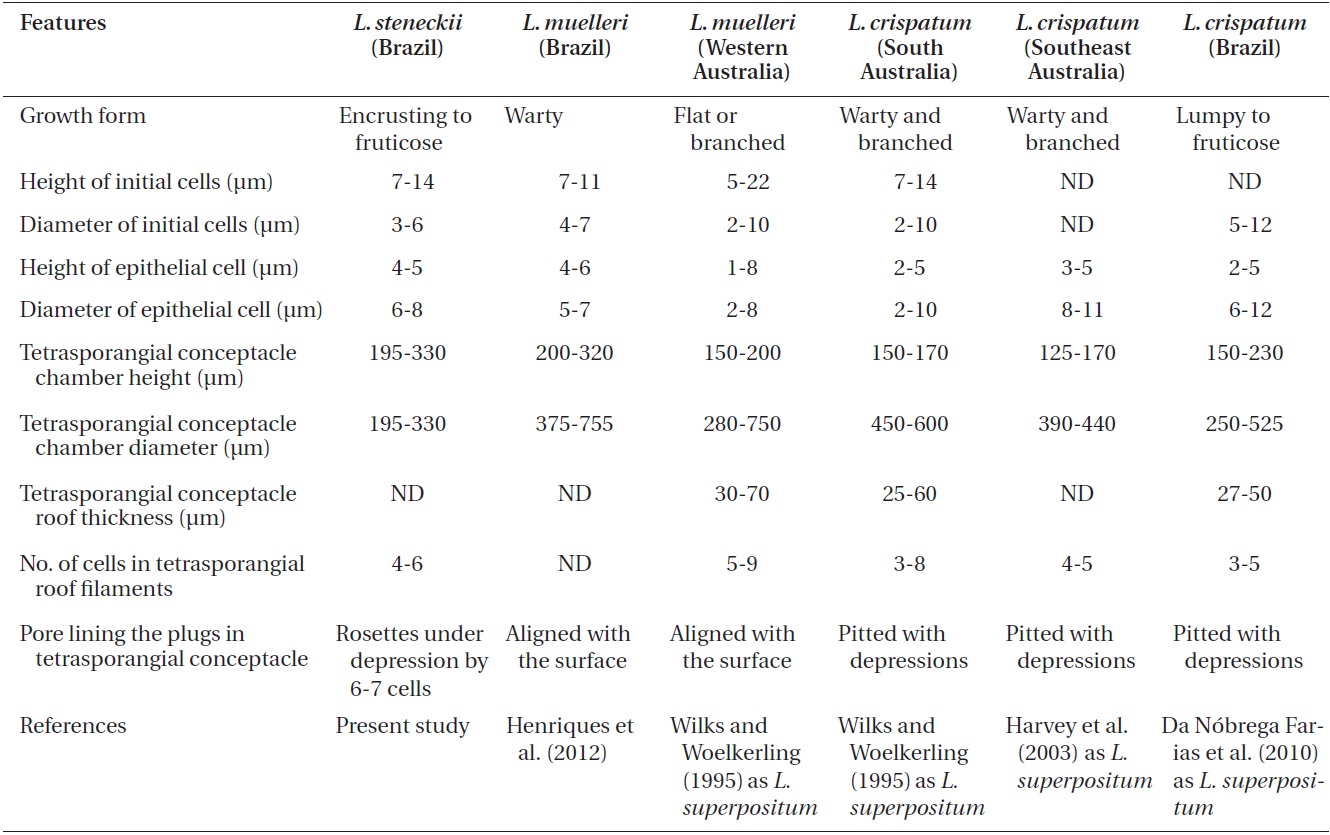
Description. Plants non-geniculate with an encrusting
growth form (Fig. 4A); crust up to 100 mm in diameter, thalli thickness 90-560 μm that do not seem to build up in layers. Dense trichocytes present in surface view (Fig. 4B) and germination disc composed of eight cells (Fig. 4C). Monomerous organization with a central area consisting of a thick layer of filaments (Fig. 4D) with cells 2-3 times as long as they are wide, 15-30 μm long and 10-15 μm in diameter and peripheral cells of 5-15 μm long and 6-10 μm in diameter. Epithelial cells are elliptical to rounded and measure 5-10 μm long and 6-10 μm in diameter, abundant cell fusions and secondary pits absent. Trichocytes are arranged in circular patches at the surface and appear as horizontal fields in vertical section, 20-35 μm long and 10-20 μm in diameter.
Uniporate tetrasporangial conceptacle chambers are elliptical, 65-90 μm long and 230-390 μm in diameter, with the roof 5-11 cells thick (Fig. 5A). The floor of the conceptacle chamber is 3-4 cells below the level of the surrounding thalli. The conceptacle roof is formed from filaments that are both interspersed among the sporangial initials and peripheral to them. The pore canal is lined by papillae cells which project slightly into the canal, and a broad central collumela is present in mature conceptacles. Tetraspores are zonately arranged, 55-60 μm long and 25-40 μm in diameter. They are restricted to the conceptacle periphery.
Female (Fig. 5B & C) and carposporangial conceptacles (Fig. 5D) are small and inconspicuous, 45-90 μm long and 90-125 μm in diameter; carposporangonial conceptacle roofs are formed only from filaments located peripheral to the fertile area. The initiation of a carpogonial conceptacle begins when groups of subepithellial initials elongate, forming a small disc of cell sat the thalli surface, which begin to shed an epithelial layer (Fig. 5B). Cells
at the centre of the disc elongated less than those at the periphery, and are more densely staining. These are the carpogonial branch initials. The cells at the periphery of the disc elongate, divide, and produce filaments that form the walls and roof of the conceptacle. The filaments forming the first few layers adjacent to the carpogonial branch initials curve inwards, and continue dividing to form the roof of conceptacle. Carpogonial branches occur across the chamber floor. Trichogynes often form a tangled mass that protrude from the pore of the female conceptacle (Fig. 5C). Carposporangia develop in carpogonial conceptacles after presumed karyogamy. Conceptacles containing carposporangia have chambers that are half dumb-bell shaped, and measure 95-110 μm long and 195-215 μm in diameter (Fig. 5D). The pore canal is limited with small filaments that projected as papillae into the pore canal. The fusion cell is discoid and curved upwards near its edge. Gonimoblast filaments are borne peripherally, and originate from the lower edge of the fusion cell. Male plants were not seen.
Ecological distribution. This is the first report of the occurrence of this species in the Atlantic Ocean. The samples were collected to a depth of 4 m. This species grows over live coral (
The taxonomic history of the genus
There are 429 species (and infraspecific) names in the database at present, of which 84 have been flagged as currently accepted taxonomically (Guiry and Guiry 2012). Only few new species of the genus, such as
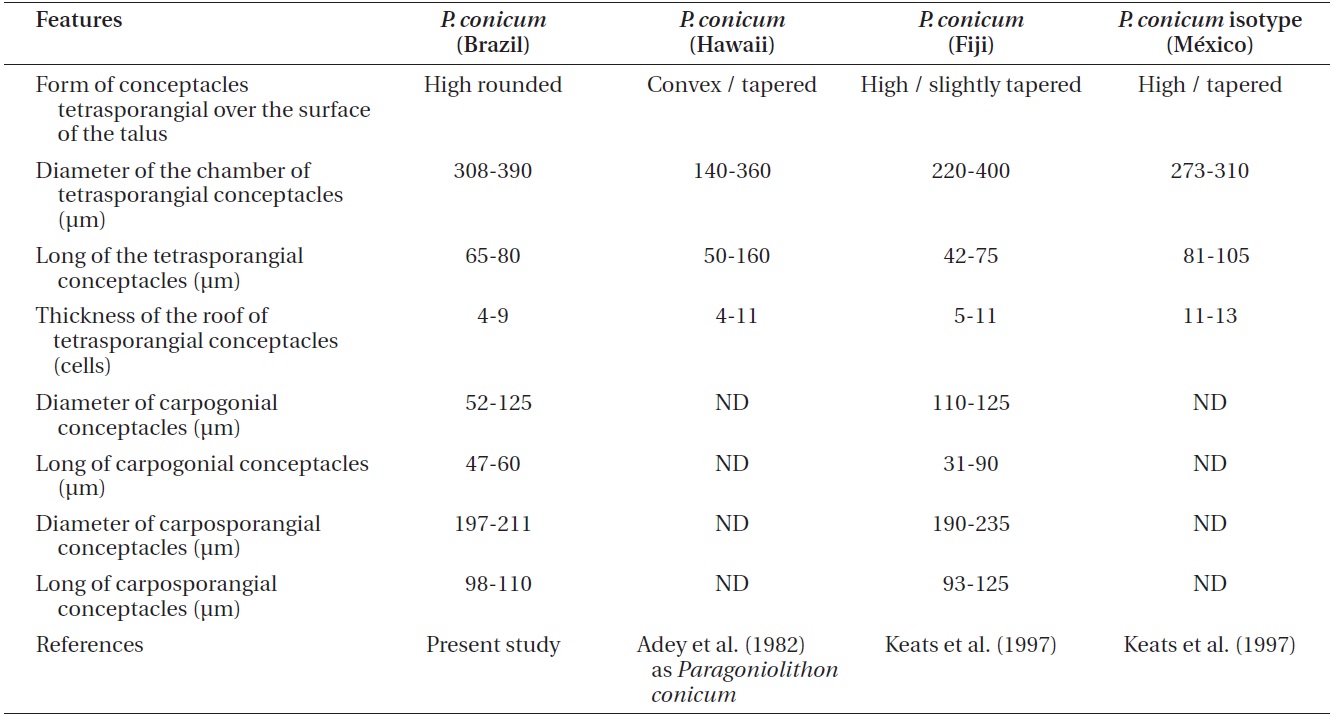
Comparison of features of Lithothamnion steneckii with closely related species reported in recent studies
Hauk (Basso et al. 2011) or represents a range extension (Henriques et al. 2012). There are a group of features reliable to delimit species within the genus.
The characteristics used to determine the species (e.g., Wilks and Woelkerling 1995, Harvey et al. 2003, Woelkerling et al. 2005, Wynne 2005, Da Nobrega Farias et al. 2010, Athanasiadis and Ballantine 2011, Basso et al. 2011, Table 1 in Henriques et al. 2012), do not allow our material of
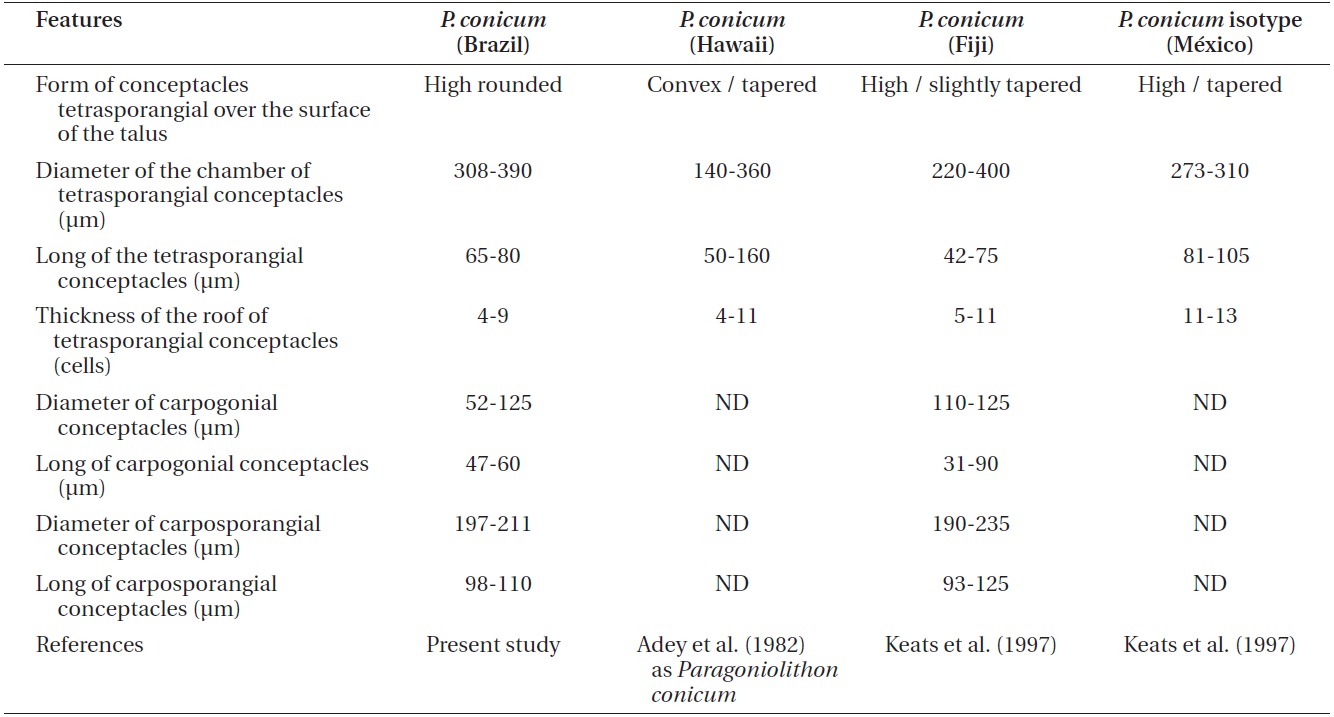
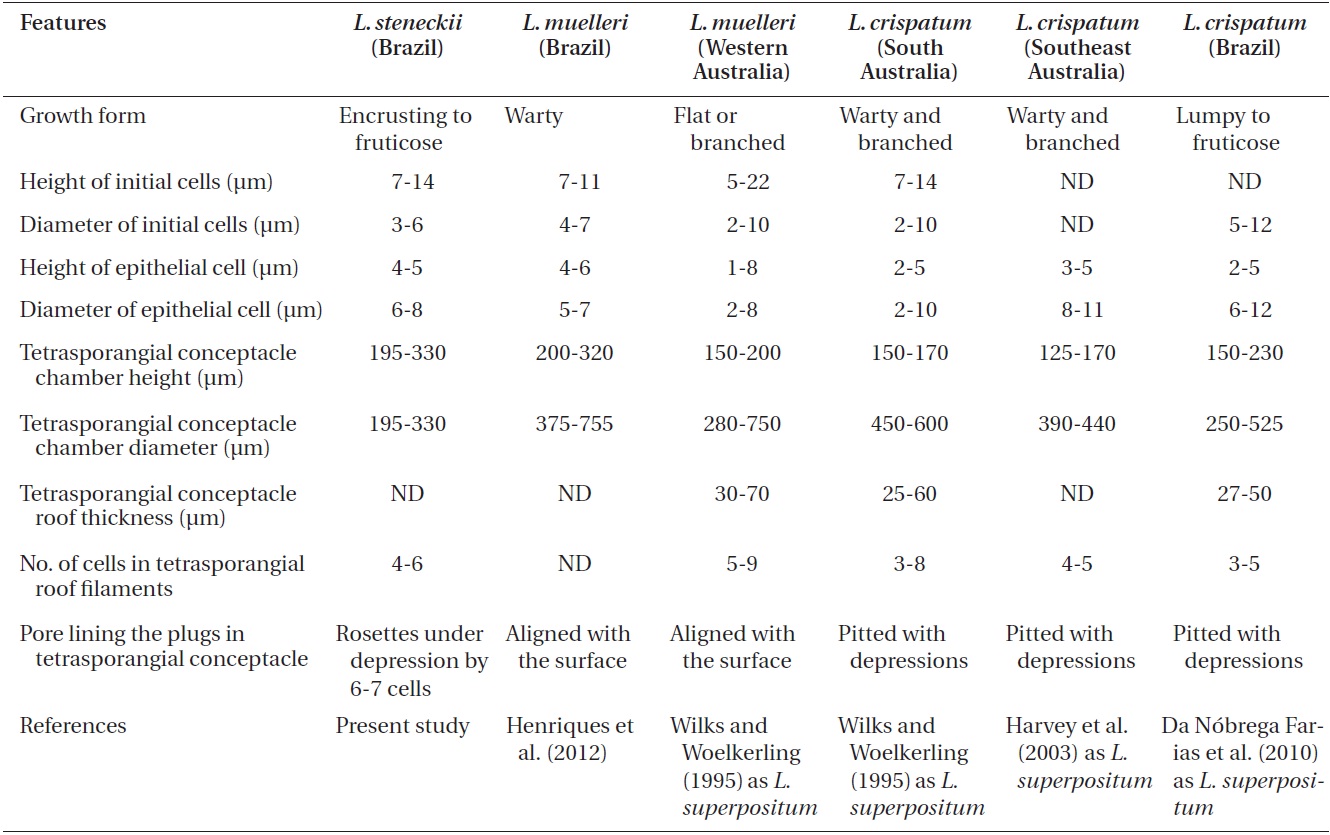
Comparison Pneophyllum conicum vegetative and reproductive anatomical features from recent studies
al. 2012) when a proper and detailed analysis is made of the coralline algae flora (Creed et al. 2010).
According to Keats et al. (1997),