The emergence and increasing spread of antibiotic-resistant microorganisms, including nosocomial and community-acquired infections with
Microorganisms are a source of antibacterial compounds; however, most are derived from terrestrial actinomycetes. Marine microbial metabolites provide the opportunity to produce novel antibiotics with unique chemical features, as compared to terrestrial ones, because marine microorganisms can live under harsh conditions such as high pressures, low food availability, total darkness, and extreme cold (Rahman et al., 2010; Abad et al., 2011). Indeed, several antibacterial compounds from marine microorganisms such as thiomarinols from
>
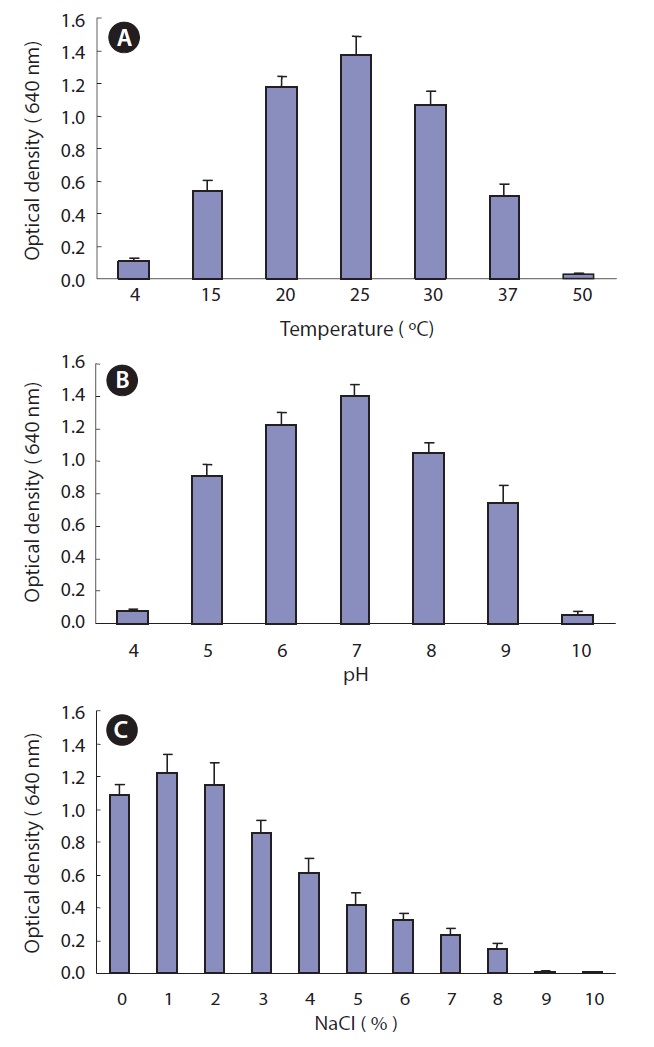
Optimum culture conditions for Pseudomonas sp. UJ-6
The optimal temperature, pH, and NaCl concentration were determined for the culture of
>
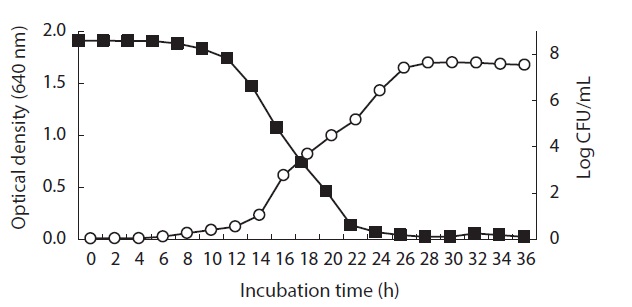
Relationship between Pseudomonas sp. UJ-6 cell growth and anti-MRSA activity
Broth from a
>
Crude isolation of the anti-MRSA substance from a Pseudomonas sp. UJ-6 culture
Isolated UJ-6 was cultured in PPES-II broth medium at 25℃ with shaking at 150 rpm for 48 h, after which the cellfree supernatant was obtained by centrifugation (15,000
The two-fold serial dilution method was used to determine the MIC of the extract as described by the National Committee for Clinical Laboratory Standards (2004). The MIC of the crude extract was defined as the lowest concentration without growth after incubation at 37℃ for 24 h.
>
Effect of the crude extract on MRSA cell morphology
To compare the effects of the crude extract on MRSA cell morphology, MRSA cells were incubated at 37℃ for 24 h in the presence or absence of the extract and then observed using a transmission electron microscope (JEM 1200EX-II; JEOL, Tokyo, Japan) at Pusan Paik Hospital (Busan, Korea).
>
General characteristics of the crude extract
To investigate the thermal stability of the crude extract, the extract was incubated at several temperatures (4, 25, 50, 75, and 100℃) for 1 h. It was also autoclaved at 121℃ for 15
min. To determine its pH stability, the crude extract was suspended in 0.1 M citrate phosphate buffer at a pH of 3 to 7 or 0.1 M Tris-HCl buffer at a pH of 8 to 10 for 30 min. After treatment, the anti-MRSA activity of the extract was estimated by the disk diffusion method.
>
Culture characteristics of Pseudomonas sp. UJ-6
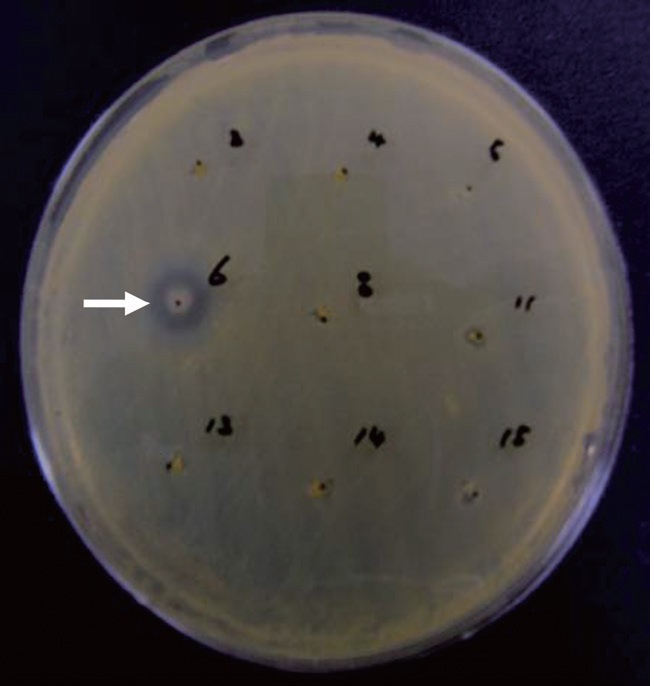
The anti-MRSA activity of
>
Anti-MRSA activity of Pseudomonas sp. UJ-6
The supernatant of cultured
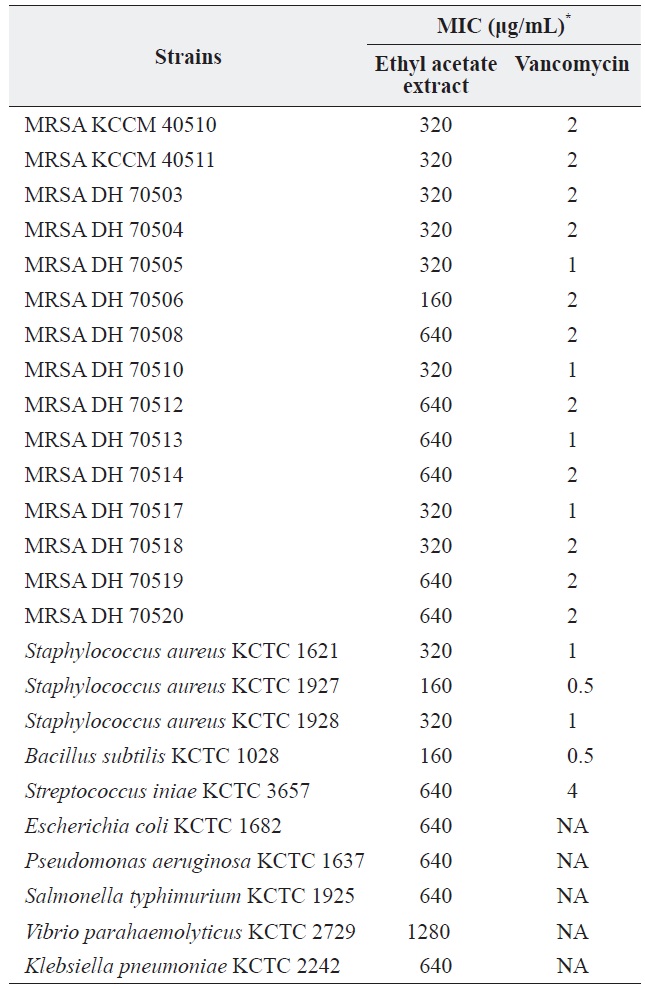
a culture was extracted with several organic solvents, including ether, hexane, chloroform, methylene chloride, and ethyl acetate. Among these, only the ethyl acetate extract showed significant antibacterial activity against all of the tested grampositive species, including MRSA strains, and all tested gram-negative species. The MICs of the ethyl acetate extract against the MRSA strains and other bacteria are shown in Table 1. The ethyl acetate extract showed antibacterial activity against the tested MRSA strains with MIC values ranging from 160 to 320 μg/mL. The extract also exhibited antibacterial activity against gram-negative bacteria, although it was less effective against gram-negative bacteria and
[Table 1.] Antibacterial activity of the ethyl acetate extract of Pseudomonas sp. UJ-6 culture
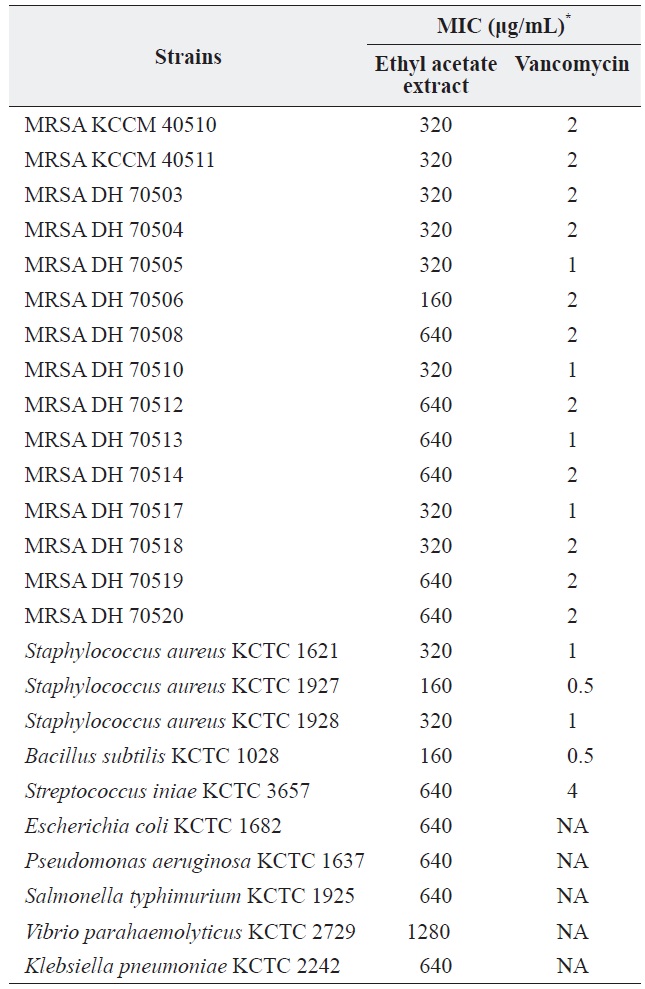
Antibacterial activity of the ethyl acetate extract of Pseudomonas sp. UJ-6 culture
>
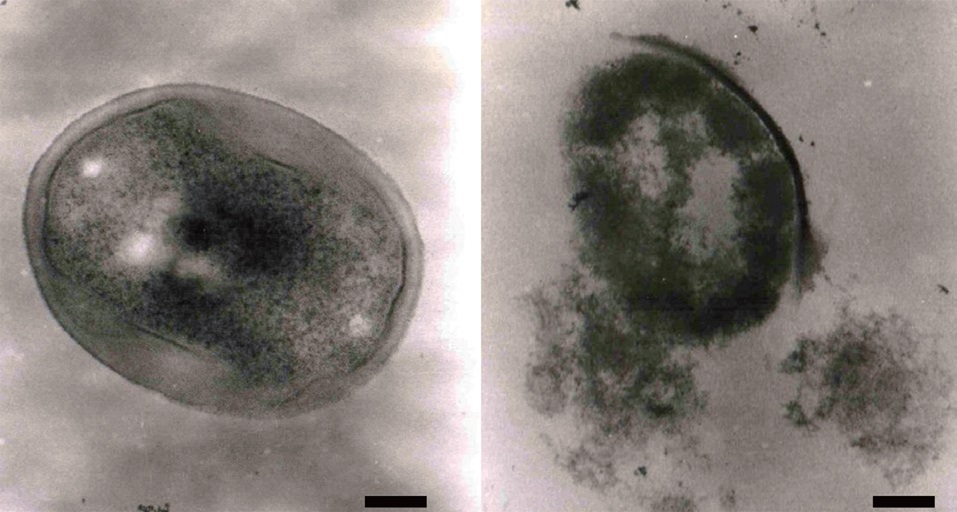
Effect of the ethyl acetate extract on MRSA cell morphology
We also investigated the morphology of MRSA cells exposed to the ethyl acetate extract using transmission electron microscopy. As shown in Fig. 4, MRSA cell lysis was observed following growth at 37℃ for 24 h with the ethyl acetate extract (320 μg/mL). Several antibiotics, including penicillin and vancomycin, interfere with cell wall synthesis, leading to cell lysis (Barna and Williams, 1984). Based on our results, we propose that
>
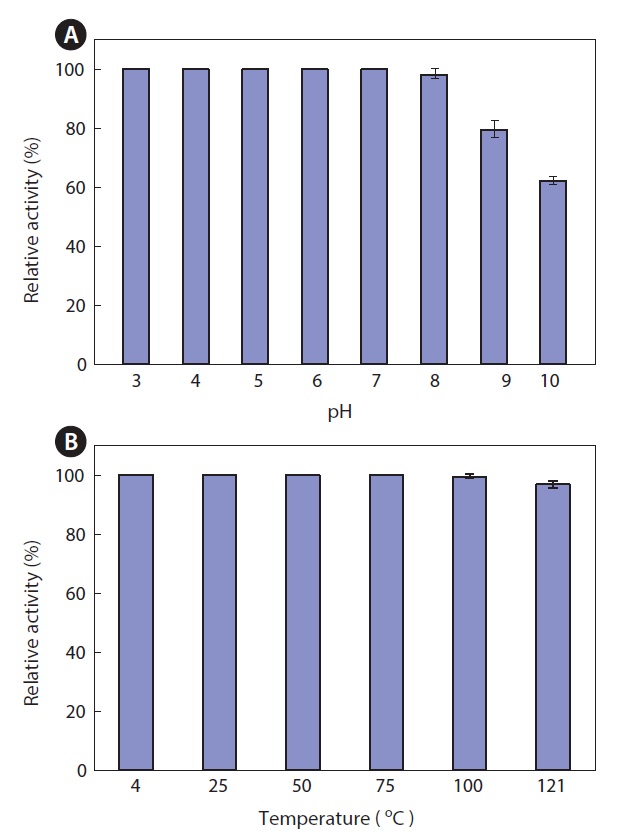
Thermal and pH stability of the ethyl acetate extract
The thermal stability and pH stability of the ethyl acetate extract were also investigated. The extract maintained >95% activity at pH 3.0-8.0, but it exhibited about 80% and 60% activity at pH 9.0 and 10.0, respectively, when the activity at pH 7.0 was defined as 100% (Fig. 5A). As shown in Fig. 5, the extract was highly resistant to thermal stress. The extract retained >95% of its activity after heat treatment for 15 min at 121℃ (Fig. 5B). This result suggests that
From these results, we anticipate that