One of the great challenges in the treatment of organic waste is related to that from the seafood and fishery industries worldwide. Prevention of marine pollution from dumping of organic waste by the 1996 London Treaty (Dyoulgerov, 1998), and other environmental legislation restricts the disposal of this waste. Discarding this waste creates two major problems: underutilization of a huge quantity of nutrients such as protein, amino acids, polysaccharides, lipids and many biologically active compounds; and the major environmental and economic problems caused by disposal of huge quantities of highly polluting organic matter. The fish?processing industry faces the need to develop efficient waste recovery and utilization methods to comply with pollution-control regulations (Choudhury and Bublitz, 1996). The processing of fish waste as resource may provide both economic and environmental benefits. The hydrolysis of waste into value-added products (protein, amino acids,
Current industrial methods of biomass waste hydrolysis include chemical (acid, alkali or catalytic) and enzymatic hydrolysis. However, chemical hydrolysis requires extreme reaction conditions and often causes serious environmental pollution. Enzymatic hydrolysis is expensive, and completion of a production cycle is time consuming. As a new-, environmentally friendly conversion method, reactions in subcritical water have gained increasing attention of late. Subcritical water can provide a medium for many chemical reactions. Changes in the physical and chemical properties of water under subcritical conditions, especially in terms of hydrogen bonding, ion products and dielectric constants, could facilitate reactions of a wide range of organic compounds, yielding many valuable materials (Yoshii et al., 2001; Laria et al., 2004; Tomita and Oshima, 2004; Yagasaki et al., 2005). Hydrolysis in subcritical water does not cause pollution and is thus an environmentally friendly technology (Cheng et al., 2008). The economic and ecological importance of thermal protein hydrolysis is increasing.
Yoshida first reported that hydrolysis in subcritical water might represent an ideal novel chemical process seafood waste treatment process (Yoshida et al., 1999, 2001, 2003; Yoshida and Tavakoli, 2004). Useful materials, such as amino acids, organic acids, fatty acids, and other materials, can be produced from fish waste by subcritical water hydrolysis (Yoshida et al., 2003; Tavakoli and Yoshida, 2006).
Food proteins have long been recognized for their nutritional and functional properties. The nutritional properties of proteins are associated with their amino acid content in conjunction with the physiological utilization of specific amino acids upon digestion and absorption (Friedman, 1996; Korhonen and Pihlanto, 2006). Amino acids are used for a variety of applications in industry, but primarily as additives in animal feeds. This is necessary, since many of the bulk components of these feeds, such as soybeans, either have low levels of, or lack, some essential amino acids. Lysine, methionine, threonine, and tryptophan are important in the production of these feeds. Antioxidant activity is thought to be of particular importance, since oxidation as an unavoidable reaction in all living organisms has been widely accepted (Halliwell, 1994). Lipid oxidation, resulting in undesirable off-flavors and potentially toxic reaction products is a significant concern in the food industry (Park et al., 2001). The aim of this study was to produce functional materials (protein, amino acids) from heatdried squid
Washed squid viscera samples were collected from F & F Co.(Busan, Korea) and transported to the laboratory on ice. Bovine serum albumin (BSA), molecular weight (MW) markers and Trolox were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). All reagents used in this study were of analytical or high performance liquid chromatography grade.
The squid viscera samples were dried in a thermal dryer at 80℃ for 24 h. The oily viscera were than filtered using a Markin cloth to remove lipids and dried again at 50℃ for 2 h.
The complete dried sample was stored at -20℃ and used for subcritical water hydrolysis. For purposes of economy, squid viscera were dried using a thermal dryer instead of a freeze dryer.
>
Proximate composition analysis
Moisture, ash and crude protein contents were determined according to the official Association of Official Analytical Chemists method (1990), and the lipid content was measured by conventional Soxhlet extraction for 24 h using hexane as the solvent. The non-protein content was estimated by subtracting the sum of the weights of moisture, ash, protein and lipids from the total weight.
>
Subcritical water hydrolysis (SWH)
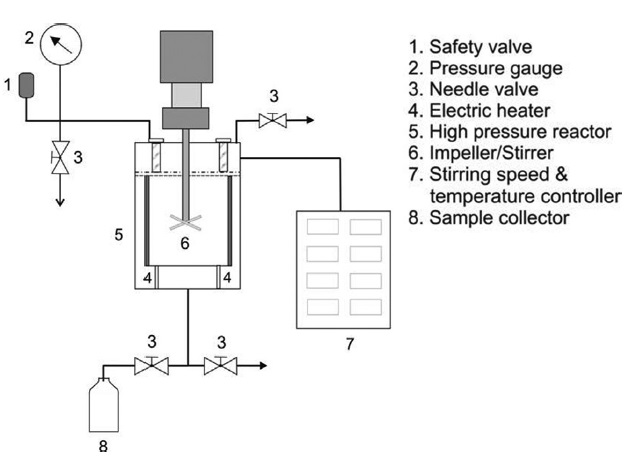
SWH was carried out in a 200 mL batch reactor composed of 276 Hastelloy with temperature control. Fig. 1 shows a schematic diagram of the SWH apparatus. Heat?dried samples (6 g) were suspended separately in 150 mL of distilled water (material to water ratio, 1:25) and charged into the reactor. The reactor was then closed and heated by an electric heater to the desired temperature (160-280℃). The pressure was estimated based on a saturated steam table to be between 6 and 64 bar for the temperature range studied. The temperature and pressure in the reactor in each experiment were measured using a temperature controller and pressure gauge, respectively. The sample was stirred with a stirrer at 140 rpm. The reaction time for each sample was 3 min. The heat-up (amount of time that a device or system requires to go from cold to the operating temperature) was 32 to 64 min. After rapid cooling to room temperature, the hydrolyzed samples from the reactor were collected and filtered using filter paper (Advantec No. 5A, Tokyo, Japan). The residual viscera powder recovered after SWH was dried and its weight (W) was measured in grams (g). The conversion yield of squid viscera, X, was calculated from the weight change of the squid viscera as follows:
X = ([Wo ? W]/Wo) × 100
where, W0 is the total amount of squid viscera introduced into the reactor. The hydrolysate was analyzed for protein, amino acids and antioxidant properties.
The protein content of the hydrolysate was determined according to the method of Lowry et al. (1951) using BSA as a standard.
>
Sodium dodecyl sulfate?polyacrylamide gel electrophoresis (SDS?PAGE)
SDS-PAGE of different hydrolysates was carried out by the method of Laemmli (1970) using a 4.4% (w/v) stacking gel and a 10% (w/v) separating gel. Hydrolysates (10 mg protein/ mL) were mixed with sample buffer at a ratio of 1:1.5 (v/v). Then 20 μL of the sample (0.08 mg protein) was loaded into each well. Electrophoresis was performed using a Mini- Protein III cell module (Bio-Rad Laboratories, Hercules, CA, USA) at a constant current (30 mA for 1.5 h). Gels were fixed, stained with 0.1% Coomassie Brilliant Blue R-250 (dissolved in 45% methanol and 10% glacial acetic acid) for 40 min and destained with 30% methanol and 40% acetic acid. The MWs of the polypeptides were estimated using MW calibration markers (Sigma Chemical Corp.).
The squid viscera hydrolysates obtained by subcritical water hydrolysis were filtered and loaded onto S430 (SYKAM) and S433-H (SYKAM) amino acid auto analyzers for free and structural amino acid analyses, respectively. LCA K07/ Li (4.6ⅹ150 mm) and LCA K06/Na (4.6ⅹ150 mm) cation separation columns, column temperatures of 37-74℃ and 57- 74℃, and buffer pH values of 2.90-7.95 and 3.45-10.85 were used for free and structural amino acid analyses, respectively. The mobile phase was 5 mM
>
Antioxidant activity measurement
DPPH free radical scavenging assay
The scavenging effects of crude methanolic extracts were determined by the method of Yen and Chen (1995) with slight modifications. Briefly, 3.9 mL of 0.1 mM DPPH solution (in methanol) were added to a test tube containing 0.1 mL of hydrolysate (10 mg protein/mL). The mixture was vortexed for 10 s and kept at room temperature for 30 min in the dark. The absorbance values of all sample solutions were measured at 517 nm. The percentage of DPPH free radical scavenging capacity (%) was calculated as [1 ? (As/Ac)] × 100 (where, As = absorbance of the crude extract at 517 nm and Ac = absorbance of the blank at 517 nm). Blank and control (1.5 mM/ standard Trolox) samples were analyzed by the same method.
ABTS free radical scavenging assay
For ABTS assays, the method of Zheleva-Dimitrova et al. (2010) with some modifications. ABTS was dissolved in water to a concentration of 7 mM. ABTS+ was produced by reacting an equal volume of ABTS stock solution with 2.45 mM potassium persulfate; the mixture was allowed to stand in the dark at room temperature for 16 h before use. For sample testing, the ABTS+ stock solution was diluted with 80% methanol to an absorbance of 0.70 ± 0.02 at 734 nm. After the addition of 3.9 mL of diluted ABTS+ to 0.1 mL of hydrolysate (10 mg protein/mL), the mixture was kept a dark environment at room temperature for 6 min. The absorbance values of all sample solutions at 734 nm were measured. The percentage of ABTS+ free radical scavenging capacity (%) was calculated as [1 ? (As/Ac)] × 100 (where, As = absorbance of the crude extract at 734 nm and Ac = absorbance of the blank at 734 nm). Blank and control (0.5 mM standard Trolox) samples analyzed by the same method.
Reducing power assay
Reducing power was determined by the method of Oyaizu (1986). Sample solution (10 mg protein/mL, 1 mL) was mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50℃ for 20 min. An aliquot (2.5 mL) of 10% trichloroacetic acid was added to the mixture, which was then centrifuged at 3,000 rpm for 10 min. The upper layer of the solution (2.5 mL) was mixed with 2.5 mL of distilled water and 2.5 mL of 0.1% ferric chloride and the absorbance at 700 nm was read. Increased absorbance indicated increased reducing power.
>
Thermal stability of hydrolysate
To determine thermal stability, hydrolysates (2 mL)were transferred to screw-capped test tubes. The tube was capped tightly and placed in a boiling water bath (100℃) for 0, 25, 50, 75, 100, 125 or 150 min. A sample without incubation (25℃) was used as a control. Residual antioxidant activities were determined by ABTS assay.
All experiments were carried out in triplicate. Data are expressed as means ± standard deviation. Differences (
>
Composition of heat?dried squid viscera
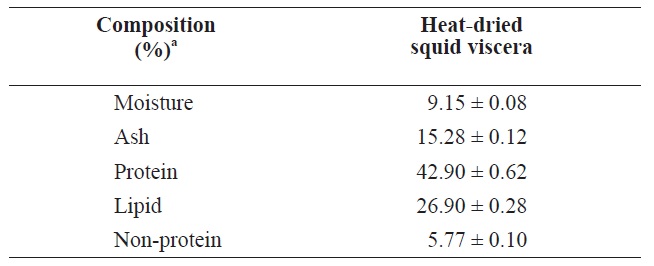
The compositions of heat?dried squid viscera are shown in Table 1. The protein and lipid contents were 42.90 ± 0.62% and 26.90 ± 0.28%, respectively, in heat?dried squid viscera.
[Table 1.] Proximate compositions of heat?dried squid viscera
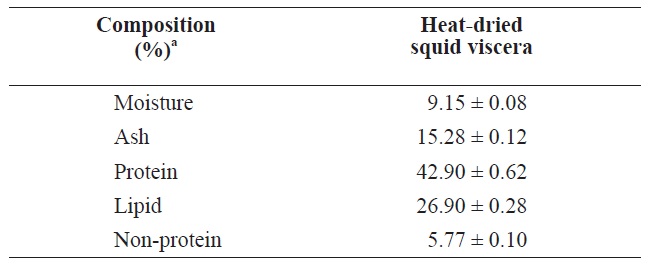
Proximate compositions of heat?dried squid viscera
Uddin et al. (2010) reported that freeze?dried squid viscera contained 45.76% protein and 39.24% lipid slightly higher than heat?dried squid viscera. These compositions may differ due to fish processing or seasonal variations in fish (Boran and Karacam, 2011).
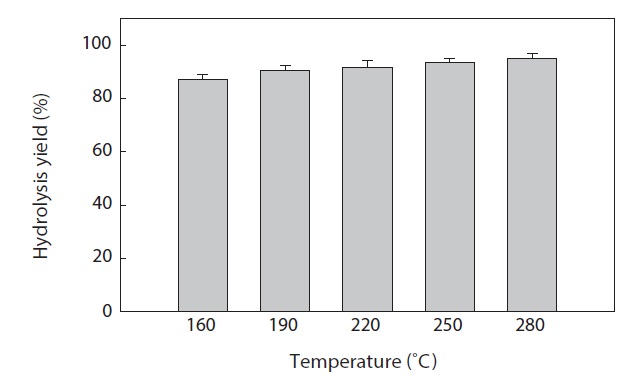
The hydrolysis efficiency (yield) at different temperatures after SWH of heat?dried squid viscera is shown in Fig. 2. Hydrolysis yield increased with increasing temperature in the vessel (high?pressure reactor). The greatest squid viscera hydrolysis yield was 95.02 ± 2.00% at 280℃. Similar results were reported by Watchararuji et al. (2008) and Park et al. (2012) for subcritical water hydrolysis of rice bran, soybean meal and
>
Protein yield in hydrolysate
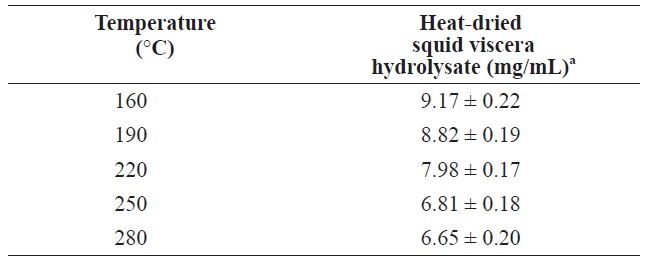
The protein contents of in squid viscera hydrolysates hydrolysates generated using various temperatures and pressures are shown in Table 2. The highest protein was 9.17 ± 0.22 mg/ mL at 160℃. As the temperature increased, the protein yield decreased. Similar patterns were reported for scallop waste, soybean and freeze?dried squid viscera (Tavakoli and Yoshida, 2006; Watchararuji et al., 2008; Uddin et al., 2010). This suggests that protein molecules decompose to water?soluble low MW organic compounds at elevated temperatures.
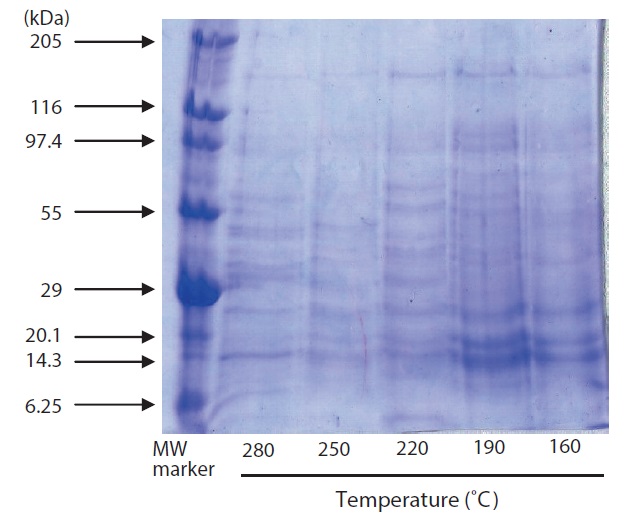
The results of SDS-PAGE of hydrolysates are shown in Fig. 3. A large number of subunits and polypeptides with masses of 97.4 to 6.25 kDa were detected in the hydrolysate by SDS-PAGE. Of the hydrolysate samples, those generated by heating to 220℃ yielded more low?MW peptide bands; fewer
[Table 2.] Protein yield from heat?dried squid viscera hydrolysates at different temperatures
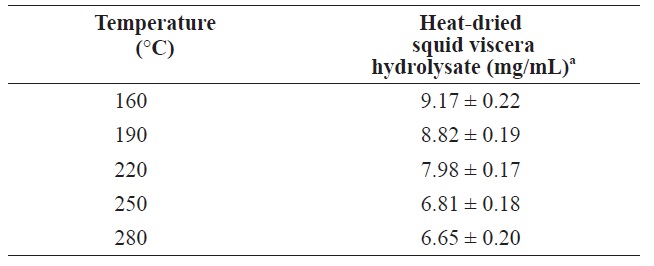
Protein yield from heat?dried squid viscera hydrolysates at different temperatures
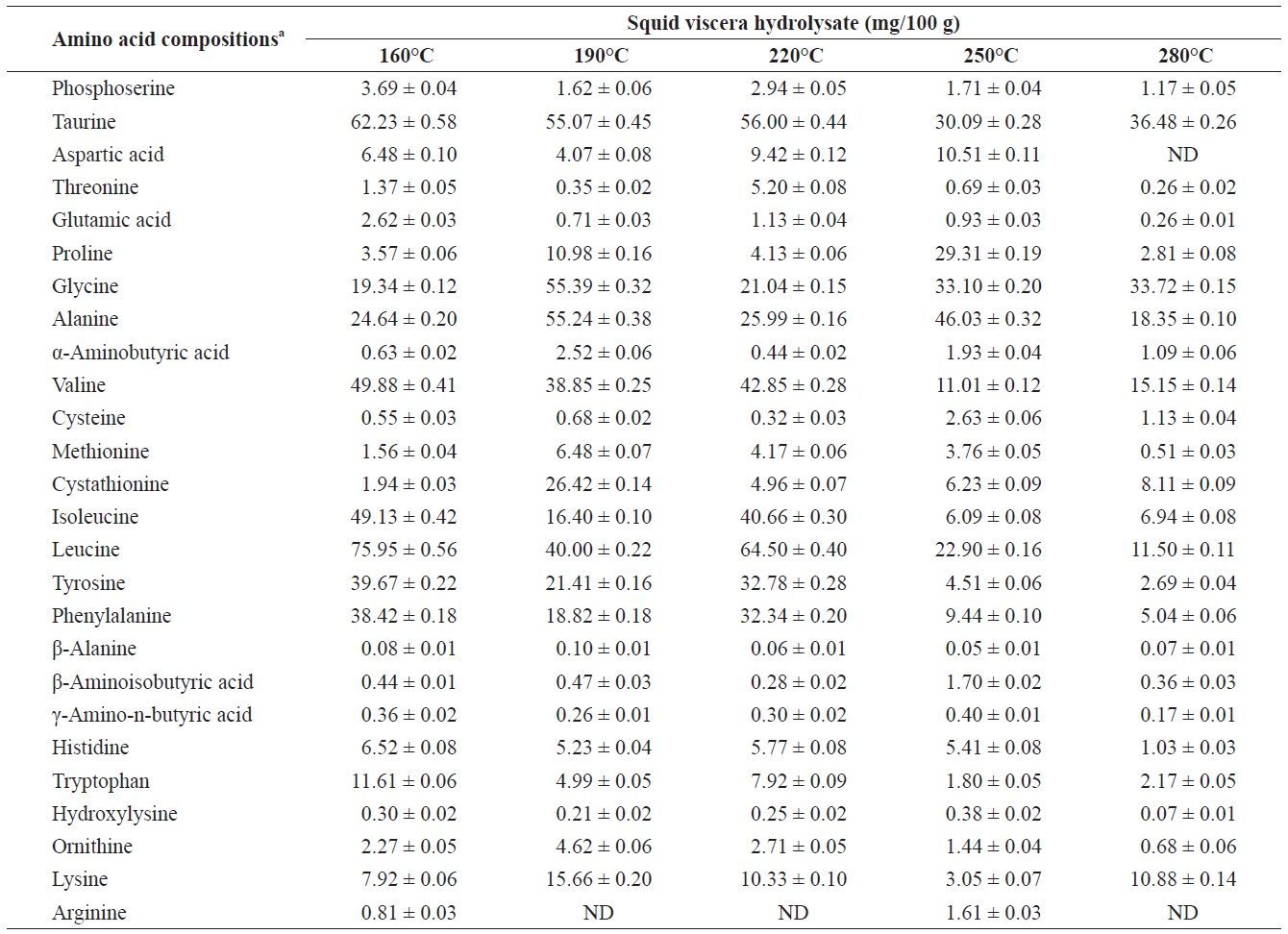
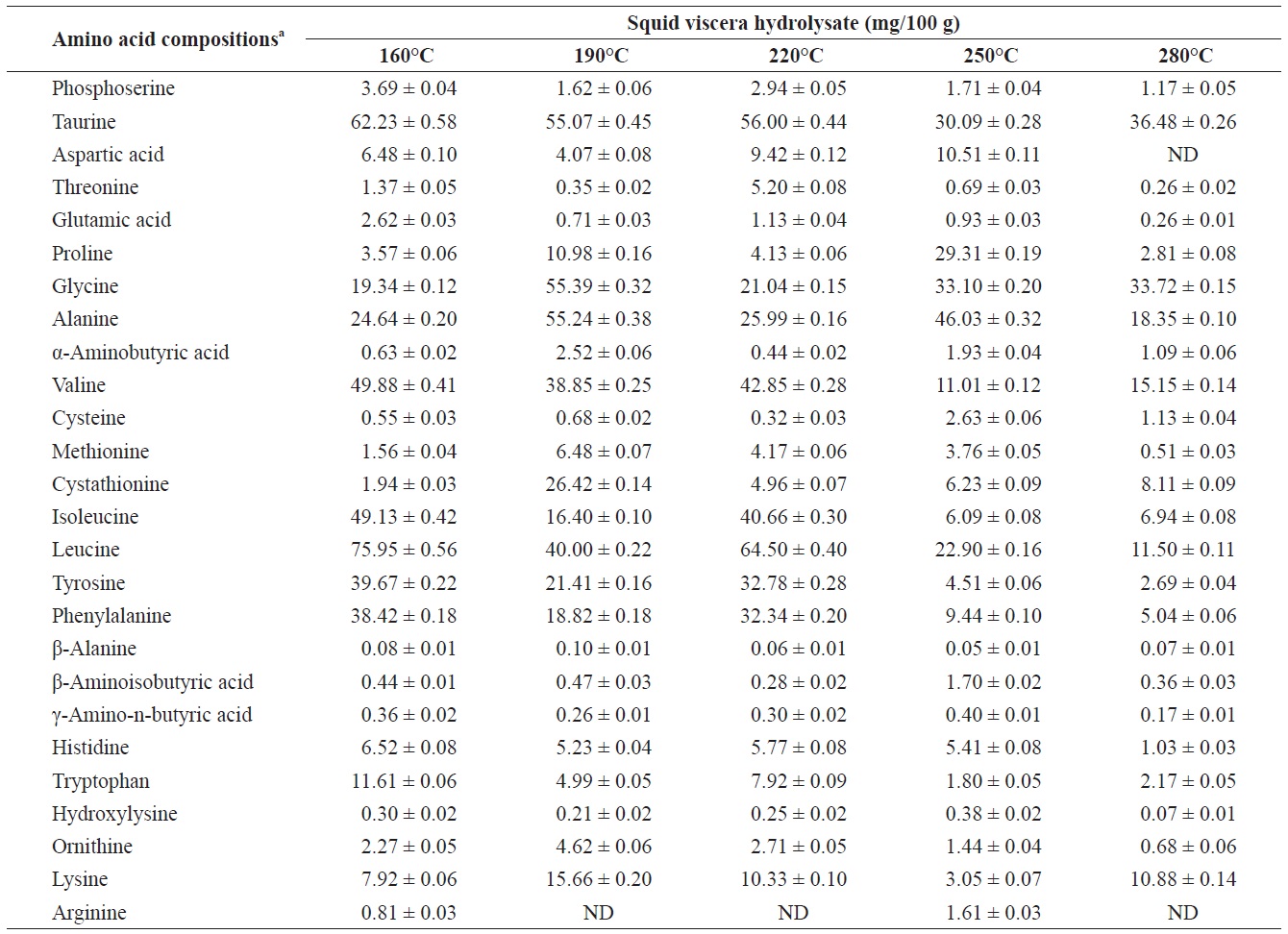
Individual composition of free amino acids yield from heat?dried squid viscera hydrolysates at different temperatures
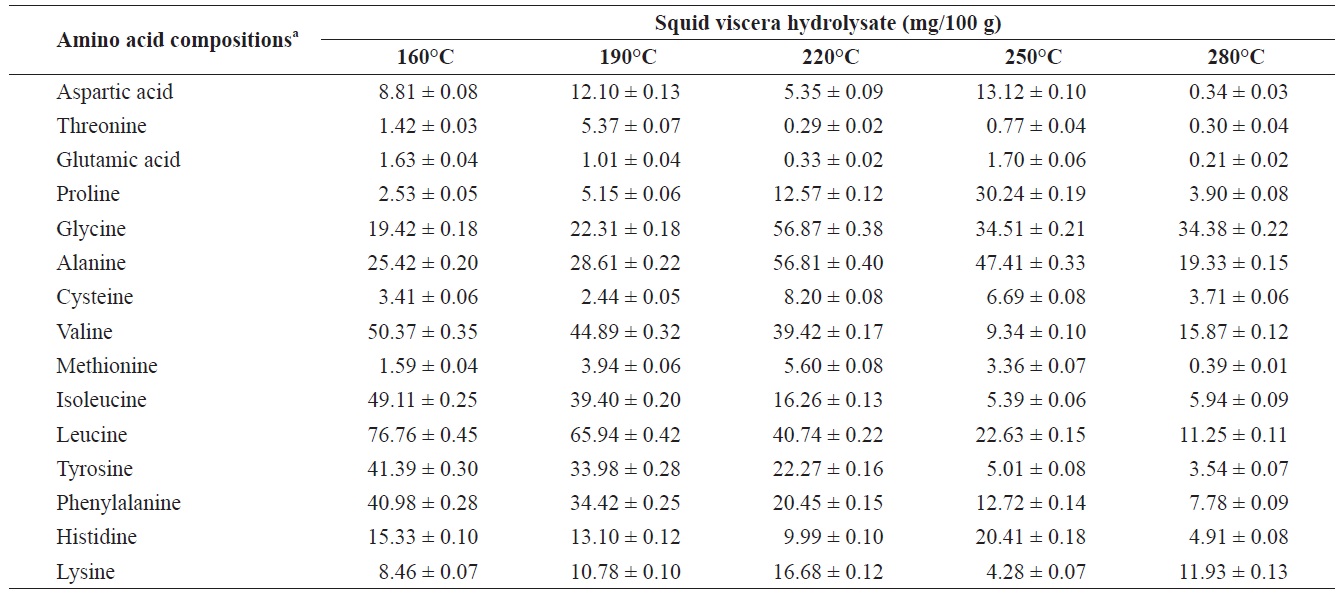
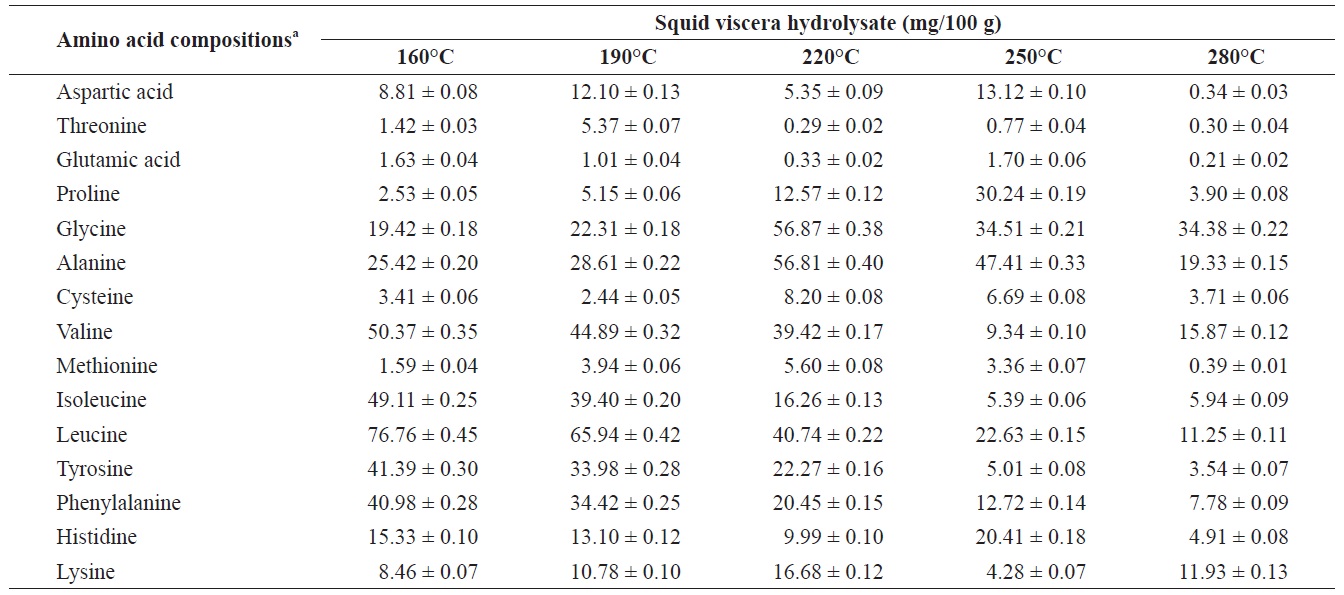
Individual composition of structural amino acids yield from heat?dried squid viscera hydrolysates at different temperatures
peptide bands were detected at higher temperatures. Similar results have been reported for scallop viscera waste (Tavakoli and Yoshida, 2006).
>
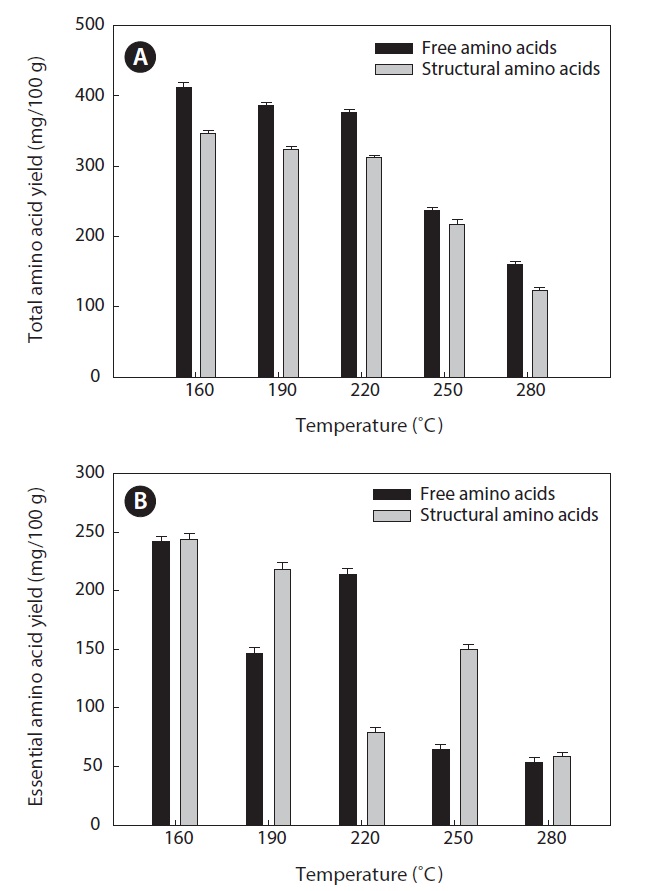
Total and essential amino acid yields
Amino acids play central roles both as building blocks of proteins and as intermediates in metabolism. Various amino acids taste sweet and bitter, and so contribute to the flavor of foods. Amino acids and protein hydrolysates are therefore useful additives in the food industry (Rogalinski et al., 2005). The total and essential (free and structural) amino acid yields of heat?dried squid viscera hydrolysate are shown in Fig. 4. Regarding total amino acids, the free amino acid yield was higher than that of structural amino acids in the hydrolysates. However, essential amino acids showed the opposite pattern. The highest yields of free and structural amino acids in the hydrolysates were at 160℃ and were 411.95 ± 1.15 and 346.62 ± 1.25 mg/100 g, respectively, for total amino acids and 242.35 ± 1.06 and 244.00 ± 1.14 mg/100 g at 160℃, respectively, for essential amino acids. The amino acid yield was reduced at 220℃. High temperatures result in decomposition of amino acids into organic acids or other products (Sato et al., 2004). Kang and Chun (2004) reported that decomposition of amino acids into organic acids or volatile materials resulted in significant decrease in amino acid production from hydrothermal processing of fish.
The yields of individual free and structural amino acids in hydrolysates generated by treatment at various temperatures are shown in Tables 3 and 4. All essential amino acids were detected in heat?dried squid viscera hydrolysates. Among the essential amino acids leucine, isoleucine, valine and phenylamine were abundant. Of the free and structural amino acids, the highest yield for individual essential amino acids were for leucine (75.95 ± 0.56 and 76.76 ± 0.45 mg/100 g, respectively), isoleucine (49.13 ± 0.42 and 49.11 ± 0.25 mg/100 g, respectively), valine (49.88 ± 0.41 and 50.37 ± 0.35 mg/100 g, respectively) and phenylalanine (38.42 ± 0.18 and 40.98 ± 0.28 mg/100 g at 160℃, respectively). One of the best?known essential amino acids is tryptophan, which helps to induce normal sleep and reduce anxiety, depression and artery spasm risk, and enhances the immune system. The yield of tryptophan in the heat?dried squid viscera hydrolysates was 11.61 ± 0.06 mg/100 g of free amino acids. At temperature ≥ 160℃, the individual amino acid levels decreased gradually and very low was at 280℃. Cheng et al. (2008) and Uddin et al. (2010) reported that the yields of most amino acids were maximum at reaction temperatures of 180 to 220℃ and 200 to 290℃. The difference compared to our results may be due to the different sample processing and experimental conditions. Other studies works reported that the thermal degradation of amino acids occurs at temperatures above 250 to 300℃, depending on the raw protein material and corresponding contact time (Daimon et al., 2001; Quitain et al., 2001; Yoshida et al., 2003).
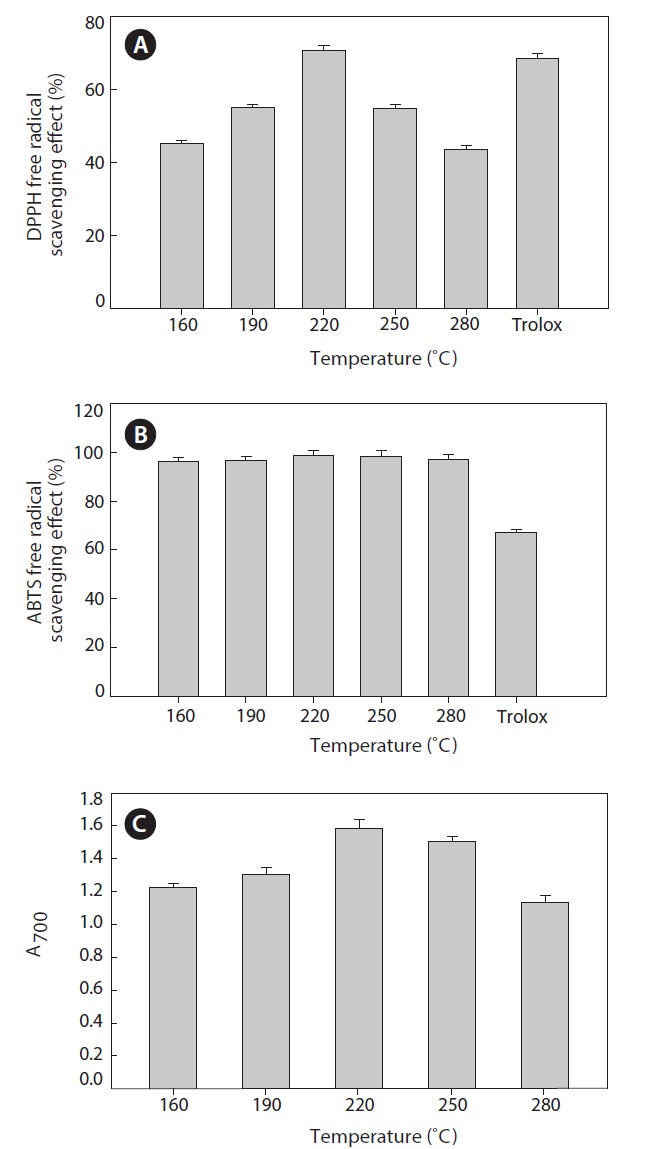
DPPH free radical scavenging effect
DPPH is widely used for free radical scavenging assessments due to its ease of use and convenience. DPPH is a stable free radical with a maximum absorbance in methanol at 517 nm. In the present study, all hydrolysates were found to be effective scavengers of DPPH radicals (Fig. 5A). The maximum scavenging effect was 70.63 ± 1.25% at 220℃. At higher temperatures, the scavenging effect decreased. When DPPH encounters a proton-donating substance, such as an antioxidant, the radical is scavenged and the absorbance is reduced (Shimada et al., 1992). Our result reveals that the heat?dried squid viscera hydrolysates might contain electron donors that react with free radicals and convert them to more stable products, terminating the radical chain reaction.
ABTS free radical scavenging effect
The capacities of the various extracted hydrolysates to scavenge ABTS radicals were determined (Fig. 5B) and found
to be similar. The hydrolysate generated at 220℃ had a slightly stronger scavenging capacity, 98.78 ± 1.80%. During hydrolysis at high temperatures and pressures, various smaller peptides and free amino acids are generated. Changes the size, level and composition of free amino acids and small peptides affect the antioxidant activity (Wu et al., 2003).
Reducing power
Reducing power is measure of antioxidant ability and may serve as an indicator of antioxidant activity. Several studies indicate that the antioxidant effect is related to the development of reductones (Yen and Duh, 1993). In the present study, all hydrolysate samples showed high reducing power (Fig. 5C).
The reducing power of the hydrolysate generated at 220℃ was markedly higher than those of other hydrolysates. At temperatures >220℃, reducing power decreased. Therefore, heat?dried squid viscera hydrolysates could next their effects by donating electrons to free radicals and high temperatures may result in decomposition of peptides to other organic compounds.
>
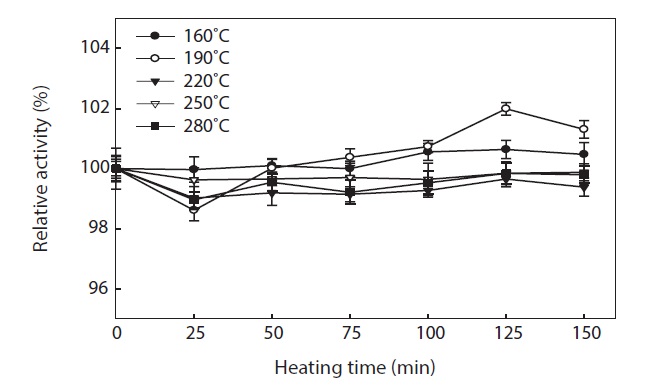
Thermal stability of hydrolysate
To generate the hydrolysates, we applied a high temperature for a short period of time (3 min). This treatment resulted in all hydrolysates having high antioxidant. The thermal stability of hydrolysates was evaluated by means of their ABTS radical scavenging activity (Fig. 6). ABTS radical scavenging activity of hydrolysates was stable during incubation at 100℃ for up to 150 min, with >98 ± 0.26% activity being retained. The small reduction in ABTS radical scavenging activity may have been due to aggregation of antioxidant peptides, by the long term heat treatment. Proteins are generally heat labile, which leads to their aggregation and exposure of hydrophobic domains (Sikorski et al., 1981). The hydrophobicity of peptides derived from many protein sources has been reported to correlate with their antioxidant properties (Faithong et al., 2010). Our results suggest that peptides with low MWs were likely stable following heat treatment. Therefore, antioxidant peptides derived from hydrolysates can be incorporated into cooked food systems without significant loss of their antioxidant activities.