Aquaculture remains one of the fastest-growing animal food-producing sectors, accounting for almost half of total food fish and substitutes for wild fish and plants (Food and Agriculture Organization of the United Nations, 2010). However, disease has been a primary constraint to the aquaculture industry, imposing severe losses on farming facilities all over the world.
Antibiotic drugs have the capacity to kill or inhibit the growth of micro-organisms. However, the use of antibiotics to control fish disease needs to be restricted due to the emergence of drug-resistant bacteria and concerns about environmental hazards and food safety (Hernandez Serrano, 2005). The future development of aquaculture greatly depends on the development of alternative feed ingredients that can provide higher resistance against pathogens.
Marine macro- and microalgae have been supplemented in diets for different cultured fish species and have been reported to have positive effects on growth performance, feed utilization, lipid metabolism, carcass quality, stress tolerance and disease resistance (Mustafa and Nakagawa, 1995; Nakagawa and Montgomery, 2007; Guroy et al., 2011). Microalgal species are of great value because of their high bioactive materials content, including polyunsaturated fatty acids, β-carotene and other pigments (antioxidants) (Cohen and Vonshak, 1991; Mahajan and Kamat, 1995; Bhat and Madyastha, 2000; Reddy et al., 2000), sulfated polysaccharides (anti-virals) and sterols (antimicrobials) (Otle? and Pire, 2001).
Spirulina has been one of the most widely used microalgal species in aquafeeds due to its high contents of protein, vitamins, essential amino acids, minerals, essential fatty acids and antioxidant pigments such as carotenoids (Nakagawa and Montgomery, 2007). Also, its immunomodulatory activity has been shown in animal experiments, which demonstrated its enhancement of phagocytic and natural killer activities (Qureshi and Ali, 1996). Several studies have been conducted to investigate the effects of spirulina on growth, nutrient utilization and immune responses of various fish species, including rainbow trout
Quercetin is a flavonoid found in fruits, vegetables and other medicinal and aromatic plants (Rupasinghe, 2008). Its antioxidant and anti-inflammatory activities have been demonstrated in
Olive flounder
Juvenile olive flounder were transported from a private hatchery (Chang-Hae Fisheries Co., Jeju Island, Korea) to the Marine and Environmental Research Institute of Jeju National University (Jeju Island). All the fish were acclimated to the experimental conditions for 2 weeks and were fed a basal experimental diet. A flow-through system was used, and sandfiltered seawater was supplied at a rate of 3 L/min. Aeration was provided by a central aeration system and air stones. Water was exchanged every 24 h to remove fecal materials. The rearing water temperature during the experiment varied from 15℃ to 19℃, and the photoperiod was maintained on a 12:12 light:dark schedule.
At the end of the acclimation period, 20 randomly selected fish (initial body weight, 2.9 ± 0.01 g) were introduced into each tank (capacity 35 L) and supplied with seawater at a flow rate of 3 L/min. Four isonitrogenous (48% crude protein) and isocaloric (17.4 MJ/kg DM) experimental diets were prepared and formulated to contain 0% spirulina
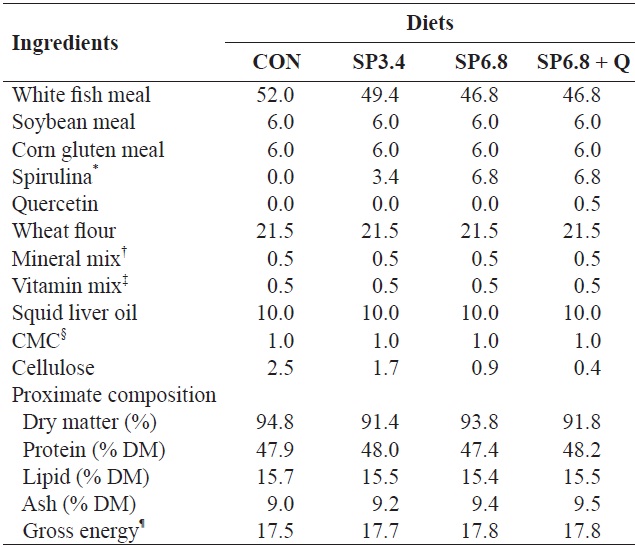
[Table 1.] Formulation and proximate composition of the experimental diets (% DM)
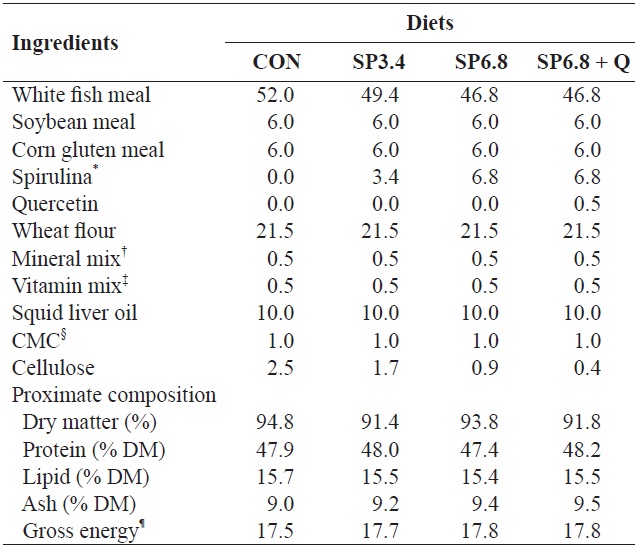
Formulation and proximate composition of the experimental diets (% DM)
were thoroughly mixed, and after addition of fish oil and 30% double distilled water, the mixture was extruded through a meat chopper machine (SMC-12; Kuposlice, Busan, Korea) in 3.0 mm diameter. The pellets were subsequently crushed into desirable particle sizes and stored at ?20℃ until use. The energy value of each diet was estimated based on mammalian physiological fuel values, i.e., 16.7 kJ/g proteins or carbohydrates and 37.7 kJ/g lipids (Lee and Putnam, 1973). The experiment was conducted in triplicate. The fish were fed at a feeding rate of 3% body mass twice daily (08:00 and 18:00 h) for 10 weeks. Fish growth was measured at 2-week intervals, and the feeding rate was adjusted accordingly. All fish were starved for 24 h and anaesthetized with MS-222 (200 mg/L) prior to handling for weighing or blood sampling.
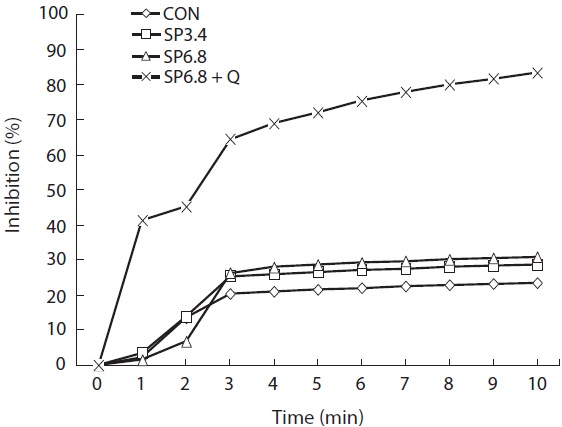
The antioxidant capacity of the experimental diets was determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, as described by Brand-Williams et al. (1995) with some modifications. Diets (2 g) were homogenized in 20 mL of aqueous methanol (80%) and kept at room temperature for 10 min. The homogenates were centrifuged (5,000 rpm, 4℃, 10 min) and filtered through a 0.45-nm syringe filter (Whatman, Inc., Clifton, NJ, USA) prior to the assay. Filtered extract (100 μL) was added to a 1.5-mL cuvette, and then 900 μL of DPPH methanolic solution (100 μM) was added to give a final volume of 1 mL. The absorbance of the mixture was recorded at 517 nm at 1-min intervals for 10 min using a spectrophotometer (Genesys 10UV; Thermo Spectronic, Rochester, NY, USA). The antioxidant capacity of the extract against DPPH radicals was calculated as percent inhibition as follows:
Percent inhibition = [(A0?As)/A0] × 100
, where A0 and As are the absorbances of the sample after 0 and S min, respectively.
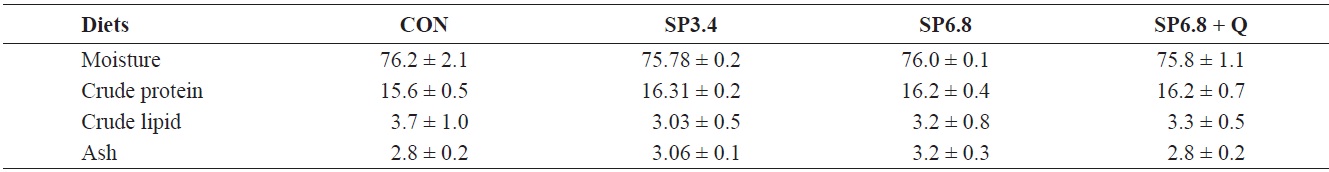
All the fish in each tank were weighed and counted at the beginning and end of the experiment for calculation of growth parameters and survival. Also, at the end of the feeding trial, blood samples were collected via the caudal vein from three randomly captured fish from each tank (nine fish per treatment) for measurement of hematological and immunological parameters. Analyses of the moisture and ash contents of the experimental diets and whole-body samples were performed by standard procedures (Association of Official Analytical Chemists, 1995). Crude protein was measured using an automatic Kjeltec 2300 Analyzer Unit (FOSS, Hoeganaes, Sweden). Crude lipid was determined using the Soxhlet Extraction System C-SH6 (Korea).
>
Hematological and immunological assays
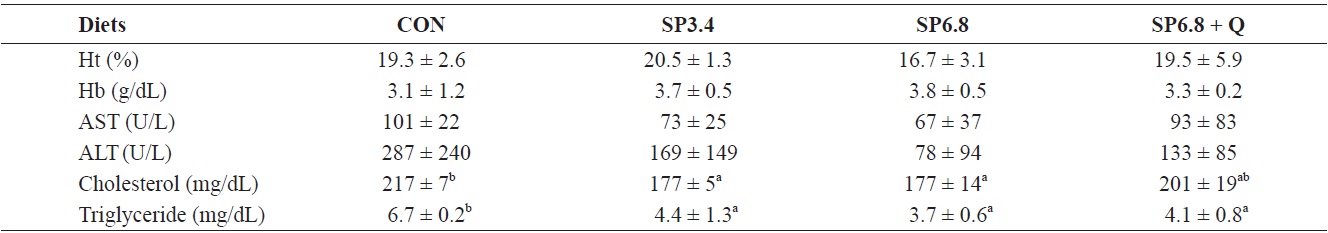
The hematocrit was determined by the microhematocrit technique (Brown, 1980). Concentrations of hemoglobin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol and triglycerides were determined using an automated blood analyzer (SLIM; SEAC, Inc., Florence, Italy).
A turbidometric assay was used to determine lysozyme activity in serum by the method described by Zhou et al. (2006) with slight modifications. Briefly,
Oxidative radical production by phagocytes during respiratory burst was measured by the nitro-blue-tetrazolium (NBT) assay described by Anderson and Siwicki (1995). Briefly, blood and NBT (0.2%) (Sigma, St. Louis, MO, USA) were mixed in equal proportions (1:1) and incubated for 30 min at room temperature. Then, 50 μL was removed and dispensed into glass tubes. Next, 1 mL of dimethylformamide (Sigma) was added, and the tubes were centrifuged at 2,000 g for 5 min. Finally, the optical density of each supernatant was measured at 540 nm using a spectrophotometer (Genesys 10 UV, Rochester, NY, USA). Dimethylformamide was used as a blank.
Data were subjected to one-way analysis of variance (ANOVA) using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). Significant differences among groups were identified by Duncan’s multiple test (
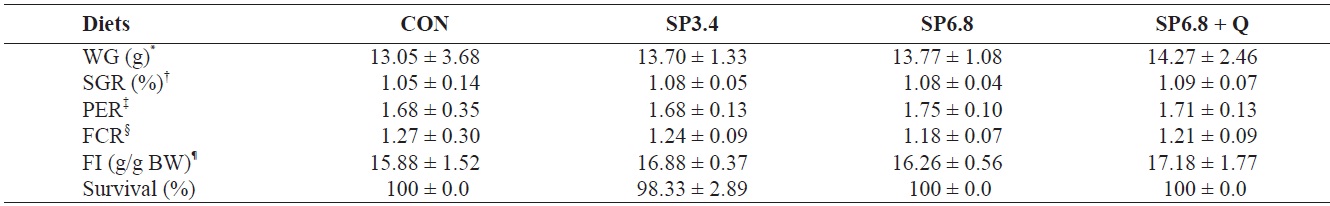
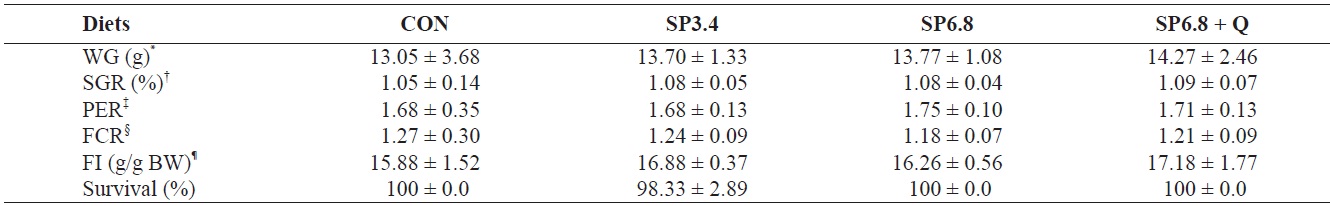
Growth performance and survival of juvenile olive flounder Paralichthys olivaceus fed the experimental diets for 10 weeks
Dietary supplementation of spirulina resulted in slightly higher antioxidant capacity, and the highest significant (
The growth performance of juvenile olive flounder fed the experimental diets is presented in Table 2. Although growth performance and feed utilization efficiency were positively affected by spirulina supplementation, no significant differences were observed among treatments. The survival rate of fish was approximately 100% regardless of dietary treatments.
No significant differences in whole-body proximate composition were detected among the experimental groups (Table 3). However, slightly higher crude protein and ash levels were found in fish fed spirulina-supplemented diets.
There were no significant effects on hematocrit, hemoglobin, AST and ALT levels (Table 4). However, the serum cholesterol level was significantly decreased in SP3.4 and SP6.8 groups compared with the control group (Table 4). Also, significant reductions were found in total triglyceride levels in fish fed spirulina-supplemented diets compared with the control group, but quercetin supplementation had no significant effect (Table 4).
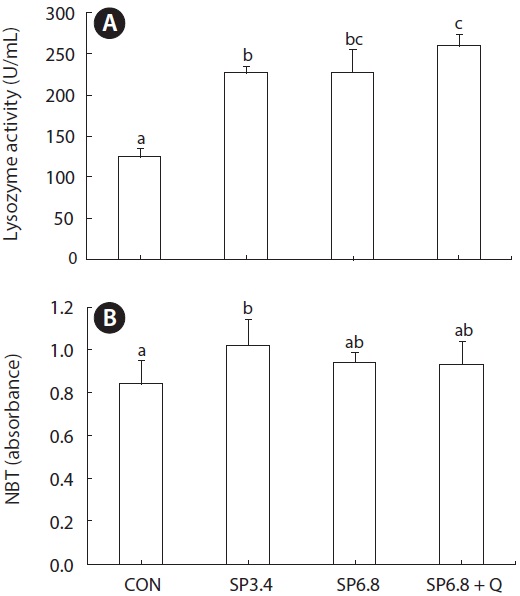
Serum lysozyme activity was significantly increased in fish fed spirulina-containing diets compared with those fed the control diet (Fig. 2A). Also, significantly higher respiratory burst activity was observed in SP3.4 group (Fig. 2B).
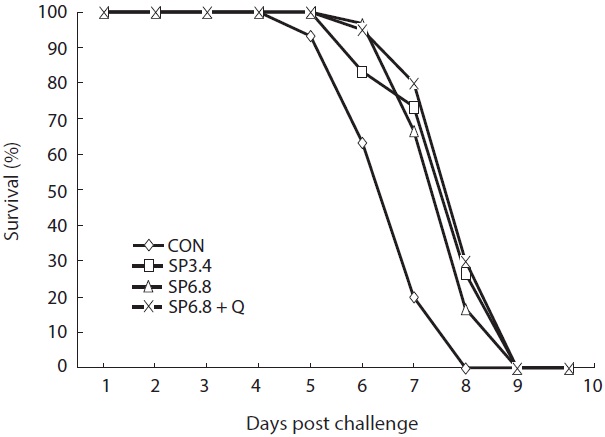
Disease resistance of the fish was not significantly affected by dietary treatments; however, the control group exhibited lower resistance against
In the present study, dietary spirulina did not significantly affect the growth performance of olive flounder. Similarly, no significant effects have been reported on growth performance in common carp
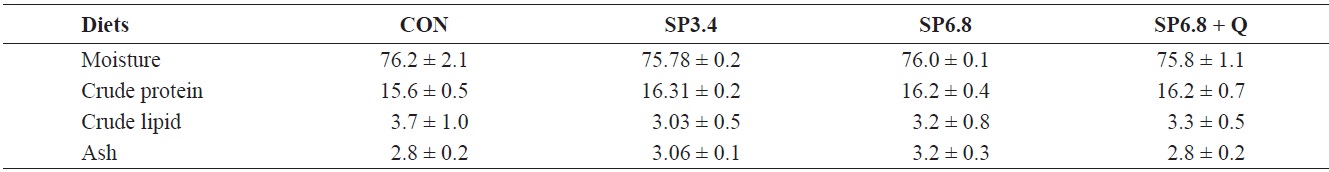
Whole body composition of juvenile olive flounder Paralichthys olivaceus fed the experimental diets for 10 weeks
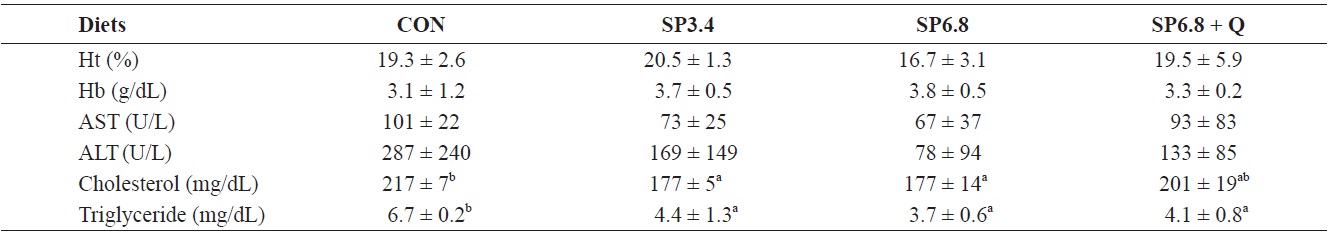
Hematological parameters of juvenile olive flounder Paralichthys olivaceus fed the experimental diets for 10 weeks
tilapia (Abdel-Tawwab and Ahmad, 2009), a red tilapia hybrid (
Abdel-Tawwab and Ahmad (2009) attributed the growth enhancement of Nile tilapia fed spirulina to improved feed intake and digestibility and suggested that high contents of several nutrients including vitamins and minerals may aid its growth-enhancing effects. Alternatively, growth improvement by spirulina may be a result of increased fish appetite (Nandeesha et al., 1998). The variation in spirulina’s effects on fish growth performance may be due to the different nutritive values of spirulina species used in experiments (Nandeesha et al., 1998). Also, Takeuchi et al. (2002) d|e|c|l|a|red that differences in the effects of spirulina on fish growth may be due to differences in the chemical compositions of experimental diets.
In the present study, the whole-body composition of fish was not significantly influenced by spirulina. As shown in the previous studies with silver seabream, Siberian sturgeon and red tilapia hybrid (El-Sayed, 1994; Palmegiano et al., 2005; Ungsethaphand et al., 2010). However, the results of some other studies revealed significant changes in whole-body crude protein (Nandeesha et al., 2001; Abdel-Tawwab and Ahmad, 2009; Promya and Chitmanat, 2011), crude lipid (Mustafa et al., 1995; Nandeesha et al., 1998, 2001; Abdel-Tawwab and Ahmad, 2009), ash (Nandeesha et al., 2001; Tongsiri et al., 2010), and moisture contents (Tongsiri et al., 2010; Promya and Chitmanat, 2011) of fish after spirulina administration.
The effects of spirulina on whole-body protein and lipid contents are correlated with their synthesis and accumulation rate in muscle, as well as the growth rate of the organisms (Smith, 1981; Fauconneau, 1984; Soivio et al., 1989; Abdel- Tawwab et al., 2006; Abdel-Tawwab and Ahmad, 2009). Nandeesha et al. (2001) stated that the effect of dietary spirulina on whole-body lipid content is dependent on species of the spirulina used. Also, its effect on whole-body lipid content differs among species (Nandeesha et al., 1998). In some studies, a raw spirulina source was used. It has been shown that rearing conditions can affect crude protein and lipid contents of spirulina, resulting in different effects on body composition of fish compared with those of commercial spirulina powder (Takeuchi et al., 2002).
It has been shown that spirulina has hypocholesterolemic and hepato-preservative properties (Khan et al., 2005; Peiretti and Meineri, 2011). Such effects have not been investigated in fish species. In this study, we examined the effects of spirulina on serum cholesterol and triglyceride levels, as well as AST and ALT levels, and found significant reductions in cholesterol and triglyceride levels. However, although the levels of AST and ALT were decreased by spirulina supplementation, no significant differences were observed among treatments.
Fish mostly rely on innate immunity in comparison to mamals (Swain et al., 2007). Accordingly, great attention has been focused on the use of dietary bioactive materials to stimulate innate immunity. The immunomodulatory activity of spirulina has been attributed to its content of C-phycocyanin (Venkataraman, 1997). In our study, significantly higher lysozyme activities were detected for both supplementation levels of spirulina, and the highest activity was observed in SP6.8 + Q group. Similarly, Promya and Chitmanat (2011) showed a significant increase in serum lysozyme activity in African sharptooth catfish
The results of challenge test showed that spirulina or quercetin supplementation could not provide protection against
Spirulina contains numerous bioactive materials including phytopigments, such as phycobilins, phycocyanin, allophycocyanin and xanthophylls, which make it a potential natural dietary source of antioxidants (Miranda et al., 1998; Bhat and Madyastha, 2000; Wang et al., 2007; Bermejo et al., 2008). In the present study, we investigated the antioxidant capacities of experimental diets supplemented with spirulina, and the results showed positive effects.
In conclusion, dietary supplementation of spirulina approximately over 3% can increase the innate immunity of olive flounder. But, the supplemental level of 3-7% is not likely to improve the growth performance of the fish rearing in low water temperature.