Sturgeons are one of the most primitive groups of Osteichthyes, and their taxonomic position relative to advanced bony fishes makes them attractive as model systems for studying the evolution and functional diversification of extant fishes (Cho et al., 2007; Kim et al., 2009; Akbarzadeh et al., 2011; Webb and Doroshov, 2011). This group of primitive ray-finned fishes includes various ploidy levels as well as several anatomical features that resemble those of cartilaginous fishes; they also exhibit distinctive morphological characters that are rarely seen in most teleostean fishes (Billard and Lecointre, 2001; Kim et al., 2005; Vasil’ev, 2009). Sturgeon undergo significant and intense morphological differentiations, especially during early life stages, and their morphogenesis during prelarval and larval stages is closely related to their behavioral patterns, such as rheotaxis, phototaxis, and swimming ability (Bolker, 2004; Kirschbaum and Williot, 2011).
Like other cold-blooded animals, environmental temperature is one of the most fundamental and critical factors affecting sturgeon development and physiology. Sturgeon species typically have their own optimum temperature range for proper egg development and larval ontogenesis, and this is one of the critical aspects that needs to be considered in hatchery management (Conte et al., 1988; Birstein et al., 1997; Gisbert and Williot, 2002). Siberian sturgeon
However, despite its potential importance, the effects of water temperature on egg development and prelarval differentiation have not been extensively studied in this sturgeon species. As a first step towards improving the capacity and efficiency of hatchery practices for Siberian sturgeon in Korea, we examined the effects of incubation temperature on the development of embryos and prolarvae, up to the transition to exogenous feeding. We also documented early ontogenic development in this sturgeon species to guide our examination of temperature-dependency in prelarval development.
>
Artificial fertilization and preparation of egg batches
To examine the effects of temperature on early development, Siberian sturgeon embryos were produced in two different hatcheries. One was from Dinoville Aquafarm Inc., Hamyang-gun, Gyeongsangnam-do, Korea, and the other was from Korea Sturgeon Aquafarm Inc., Miryang City, Gyeongsangnam-do, Korea. Three egg batches were produced from independent matings in the first hatchery; two egg batches were produced at the second hatchery. For hormonally induced spawning, mature females (8-10 years old) were given intramuscular injections of a luteinizing hormone-releasing hormone analogue (des-Gly10, [D-Ala6] LH-RH Ethylamide; LHRHa; Syndel Laboratories Ltd., Vancouver, BC, Canada). The dosage was 100?150 μg/kg body weight (BW), and 10% was delivered as a primary dose and the remaining 90% was given as a resolving dose 12 h after the primary injection. Males were given a single injection of LHRHa, at a dosage of 100 μg/kg BW. Ovulated eggs were artificially inseminated using the wet method, according to previously described procedures (Gisbert and Williot, 2002; Park, 2012).
>
Documentation of early ontogenic development
To examine morphological changes in hatchlings, hatched prolarvae were reared in a water recirculating system at 18 ± 0.5℃. After the evacuation of the pigment plug, larvae were fed with
>
Examination of temperature effects on the development of embryos and prolarvae
Three successive experiments were performed to examine the effects of temperature on egg development, hatching, and prolarval development. First, embryonic development in at least 330 fertilized embryos from each egg batch was examined under four temperature treatments (12℃, 16℃, 20℃, or 24℃). Experimental temperatures were maintained within ± 0.5℃ using electric thermostat-assisted temperature controllers. For each temperature group, the time to reach various developmental stages (blastulation, gastrulation, small yolk plug formation, late neurulation, s-heart formation, and the first occurrence of hatching) was monitored. Descriptions of each developmental stage in this species referred to Park et al. (2013). During each of the four selected stages, the survival rate (percentage) was also estimated for each egg batch. Second, the time spectrum of hatching events was examined under three different incubation temperatures (12℃, 16℃, and 20℃). Prehatching embryos (at least 200 embryos from
each group) that had developed under normal conditions at each temperature until the tail-beating stage were assigned to one of three new incubators (adjusted again to 12℃, 16℃, or 20℃). Afterward, hatched prolarvae were scored within a 24-h interval until hatching was complete. The completion of hatching in each group was defined as the absence of continued hatching over a period of 48 h. Two replicates were used for each temperature group. Third, temporal patterns in the evacuation of the pigment plug in prolarvae were examined under three temperature conditions (12℃, 16℃, and 20℃) to evaluate the effects of incubation temperature on the transition of prolarvae to the external feeding stage. Immediately after hatching, prolarvae were collected from each temperature group and transferred to one of three new tanks that were also adjusted to 12℃, 16℃, or 20℃. The percentage of larvae that evacuated their pigment plugs was determined daily using a random sample of 36 individuals from each group. Accumulated mortality was also monitored daily and the survival rate from just after hatching until the completion of evacuation was compared among the three temperature groups. Two replicate groups were created for each temperature group.
Statistical evaluations for the differences in developmental progress, hatchability, viability and rate of pigment plug evacuation were carried out using ANOVA followed by Duncan’s multiple ranged tests using the SAS version 10. 3 (SAS Inc., Cary, NC, USA). Differences were considered to be significant when
>
Morphological appearance during ontogenic development in prolarvae
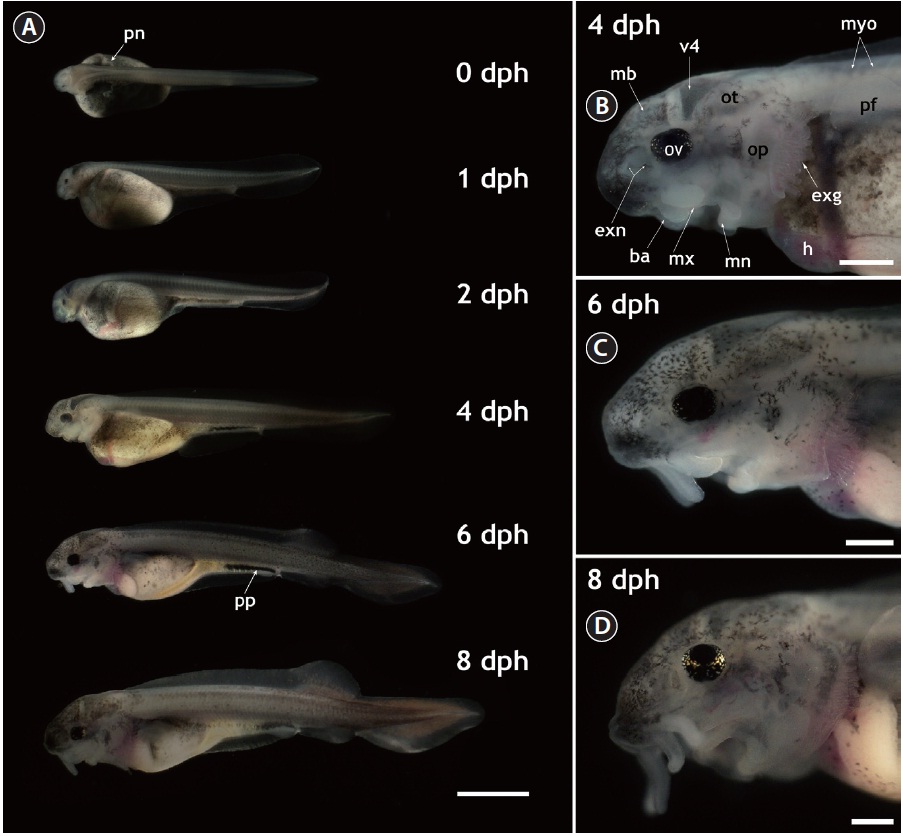
The gross morphology of yolk-bearing prolarvae from hatching to 8 dph is provided in Fig. 1. On the day of hatching (within 12 h after hatching), larvae had a large, yellow yolk sac and a straight shape. Pairs of distinct veins were visible on the surface of the yolk and the pigment plug was evenly spread inside the digestive tract. Pronephros ducts were still visible. Muscle segments could be clearly visible and each one could be distinguished from the others. Very little pigmentation in the embryonic body was observed in most prolarvae. At 1 dph (36-40 h after hatching), the eye pigmentation was more pronounced and the fourth ventricle (i.e., one of the four connected cavities in the brain) appeared dark. Until 2 dph, growth in length was not significant and average length was 11 ± 1.5 mm; however, the yolk was slightly absorbed and the head structure became more evident. Rudimentary barbels were visible on the prolarvae. After 2 dph, the prolarvae started to undergo noticeable morphological transformations. Prolarvae at 4 dph (total length [TL], 14 ± 2.4 mm) displayed significant pigmentation in the head region and on the abdominal cavity. The yolk sac was bipartite, with a furrow that formed the anterior stomach rudiment and the posterior midintestine. The pigment plug became concentrated and moved to the posterior part of the digestive tract. Pectoral fins budded on the yolk sac and were clearly visible. In the cephalic region, prolarvae at 4 dph showed extended external gills that were generally not covered by the operculi. Fully pigmented optic vesicles and well-developed eye lenses were apparent. External nares with two connected holes were visible and the barbels were elongated, allowing the clear identification of each barbel. In addition, the midbrain, the fourth ventricle, and the otocysts could be distinguished in the lateral view. At 6 dph (TL, 18 ± 3.1 mm), the prolarvae had more pigmentation over almost their entire bodies, especially in the cranial and caudal sections. The posterior margin of the yolk sac had been markedly reduced, the yolk sac had separated into presumptive organs, and organs in the abdominal cavity could be visualized. The dorsal fin was separated from the fin fold and rudimentary fin rays extended from the fin base. The anal and pelvic fins also began to appear as ridges in the fin fold. Meanwhile, the median fin anlage was evident in the caudal part with pigmentation. These prolarvae had more elongated barbels and distinctly developed operculi in the cephalic region. However, the external gills were still obvious during this stage. When the larvae reached 8 dph (TL, 20 ± 2.7 mm), the yolk sac was difficult to discern clearly. Fin folds were more developed, the dorsal fin base was clearly visible and the anal and pelvic fins were evident. The heterocercal structure of the caudal fin was clear. The pigment plug had moved further in the anal direction and eventually started to be evacuated, indicating that the larvae were transitioning to exogenous feeding. Evacuation was first observed from 8 dph and continued until 10 dph. In the lateral head view, the forth ventricle at 8 dph was less contrasted than in earlier larvae due to the thickened cranial cover, which had significant pigmentation on its surface. Barbels were much more elongated. Although the external gills that had been prominent in earlier larval stages were largely covered by the extended operculi, the coverage was not yet complete. The gills were completely covered by the operculi at 20 dph.
>
Larval development after yolk sac absorption
After the transition to exogenous feeding (
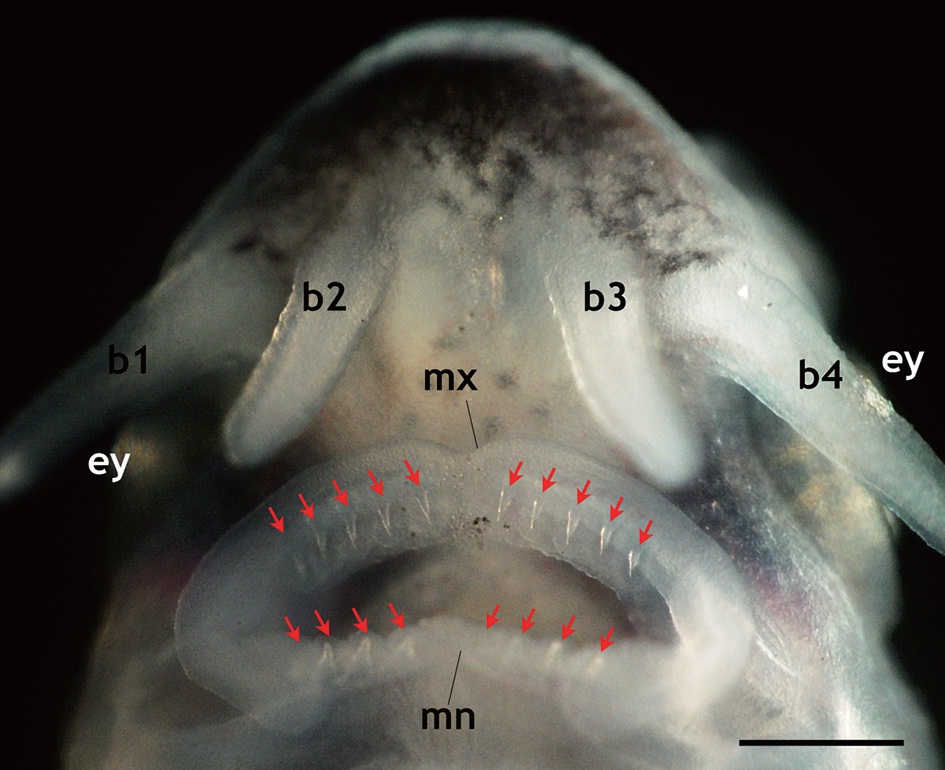
that is seen in adult fish, including five arrays of scutes, a heterocercal caudal peduncle, and highly elongated barbels (Fig. 2). During development, the larvae exhibited transient occurrences of teeth. Tooth rudiments were first seen at 5 dph in both the upper (maxillary) and lower (mandibular) layers (Appendix 1). The teeth lengthened and sharpened with age, and a shark tooth-like shape peaked at 9 and 11 dph (Fig. 3). Upper teeth were sharper and more tapered than lower teeth. However, the numbers of both upper and lower teeth were not uniform among individuals, with the number of upper teeth in most individuals ranging from 8 to 10 and the number of lower teeth ranging from 6 to 8. Afterward, these teeth began to degenerate. The upper teeth became thinner while the lower teeth took on a blunted shape; both upper and lower teeth became shorter. During 15-17 dph, the teeth were less than half the size of those observed at 9-11 dph. At 19 dph, the teeth were only vestigial and they had disappeared completely by 21 dph (Appendix 1).
>
Embryonic development and yolk sac absorption under different temperature conditions
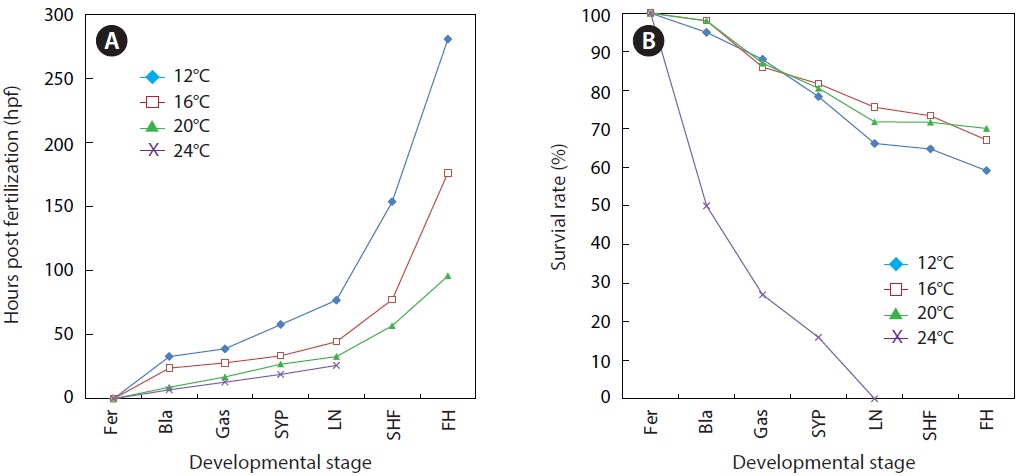
As expected, developmental speed was significantly and negatively affected by incubation temperature, and the difference in developmental speed was more significant in later developmental phases (Fig. 4A). As a result, the time to reach the point of small yolk plug formation ranged from 19 h (at 24℃) to 58 h (at 12℃). Percent viability (78-81% in average) at this stage did not differ significantly among the groups that were incubated at 12℃, 16℃, or 20℃. However, the embryos that were incubated at 24℃ showed very low survival (16%), and none of the embryos survived until the completion of neurulation. Only a few abnormal embryos reached neurulation, but all of them died (Fig. 4B). The time to reach the end of neurulation ranged from 33 h (20℃) to 77 h (12℃), and s-heart formation was observed at 57 h (20℃), 77.5 h (16℃), and 154 h (12℃). The first occurrence of hatching in the group incubated at 12℃ was observed at 281 h, while hatching first occurred at 176.5 h and 96 h in those incubated at 16℃ and 20℃, respectively. The percent survival in each of the temperature groups until the first occurrence of hatching was 59 ± 3.8% (12℃), 67 ± 4.0% (16℃), and 70 ± 1.8% (20℃). The survival rate was significantly lower at 12℃ than at the other temperatures (
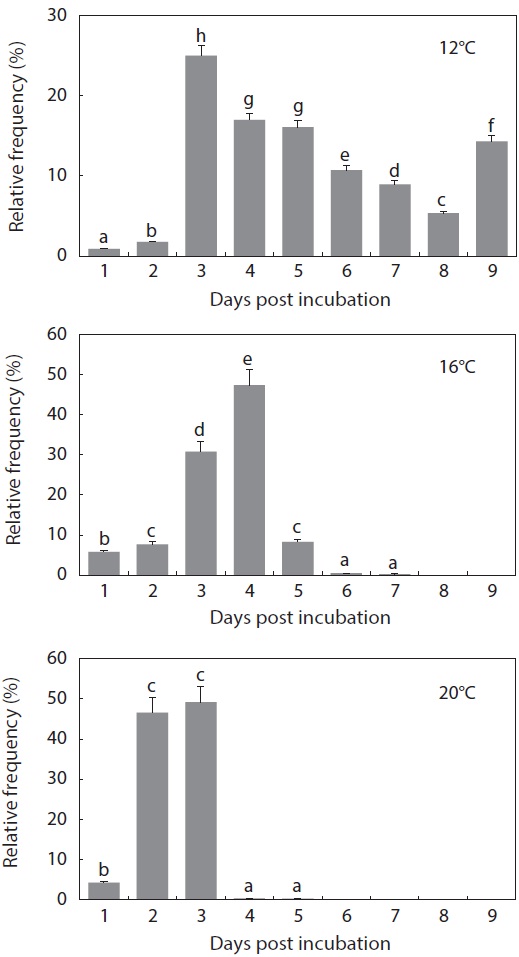
The period from the first occurrence to the completion of hatching was also affected by incubation temperature (Fig. 5). At 12℃, hatching was completed on Day 9 from the initial incubation of tail-beating embryos (
what was observed in the other temperature groups (
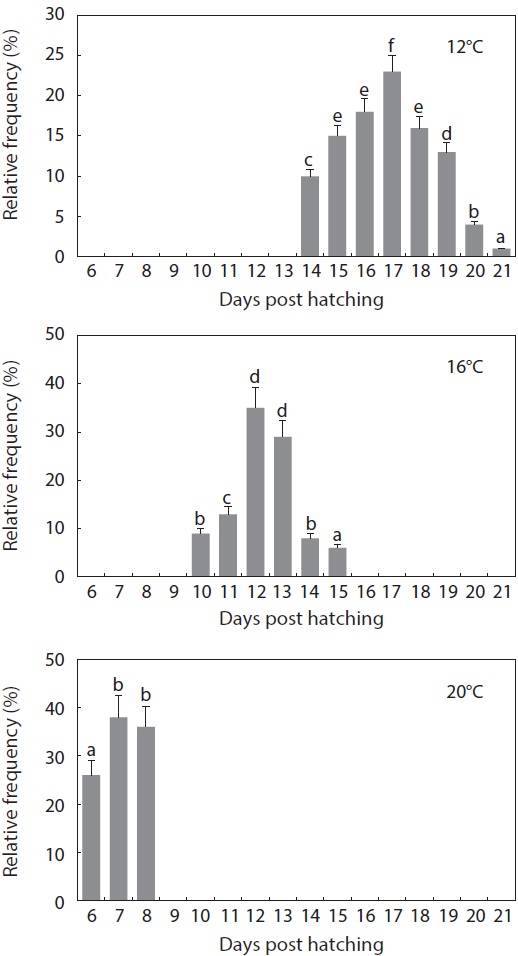
The gross pattern of morphological differentiation in yolkbearing prolarvae, as characterized above, did not differ significantly among the temperature treatments, although temporal progress was inversely related with incubation temperature. Time to yolk sac absorption in prolarvae was significantly affected by incubation temperature (Fig. 6). At 12℃, evacuation of the pigment plug was first observed at 14 dph and it continued until 21 dph. On the other hand, pigment plugs began to be evacuated at 10 dph and 6 dph, and was completed at 15 dph and 8 dph, in the 16℃ and 20℃ treatments, respectively. The average survival rate from hatching to complete evacuation of the pigment plug was significantly lower at 12℃ (68 ± 5.8%) than at the other temperatures (81 ± 9.1% at 16℃ and 84 ± 6.0% at 20℃) (
Early ontogenic development in Siberian sturgeon prolarvae and larvae was documented, and the effects of temperature on early development were examined. Overall, the spatial and temporal patterns of larval morphogenesis documented in this study were congruent with other
Meanwhile, the present study provided new details about the transient development of teeth during ontogenic development in Siberian sturgeon larvae. To date, temporal patterns in tooth development during larval stages in sturgeons have not been comprehensively studied. In this study, temporal changes in tooth development, as well as morphological features, were newly characterized. Siberian sturgeon prolarvae developed tooth rudiments as early as 5 dph and the development of shark-like teeth peaked during the period from 9 to 11 dph; afterward, the teeth degenerated progressively and completely disappeared by 21 dph. Individual variation was found in the numbers of teeth in both the maxillary and mandibular layers. Although the mechanism or biochemical signaling that is responsible for the development and degeneration of transient teeth in sturgeons is not yet understood, it is thought that the teeth are associated with the seizing and fragmentation of exogenous food (
In the temperature experiments, fertilized Siberian sturgeon embryos developed normally at three of the experimental temperatures (12℃, 16℃, and 20℃), but embryos incubated at 24℃ failed to development from the gastrulation stage. Bearing in mind that at least a portion of the embryos that were incubated at 24℃ showed successful development during the early cleavage phase, the heat-mediated problem would manifest during the formation of germ layers (
More importantly, the incubation of Siberian sturgeon embryos at temperatures close to 20℃ could provide advantages over low-temperature incubation, because there was a much more synchronized timing window for hatching events. At 20℃, the hatching events of almost all of the tail-beating embryos were completed within only 3 days. Lower incubation temperatures resulted in significantly lagged periods: at least 5 and 9 days at 16℃ and 12℃, respectively. Furthermore, hatchability was significantly higher at 20℃ than at the two lower temperatures, without any notable signs of additional abnormalities. This synchronized pattern at 20℃ has important implications for hatchery practices; it could help managers make more accurate decisions about the timing of initial feeding. Under hatchery conditions, large variation in the timing of hatching inevitably leads to non-uniform larval populations, which consequently results in the unavoidable and unwanted loss of a portion of the larvae that show either advanced or delayed development. It is widely agreed that an earlier supply of artificial food, before the complete transition to exogenous feeding, would not provide any advantage to larvae, while a late food supply would significantly depress both growth and viability (Gisbert and Williot, 1997). Moreover, a considerable portion of the embryos that were incubated at the lower temperatures (particularly at 12℃) did not hatch and eventually died, although they appeared to be morphologically healthy. Dead embryos would cause bacterial and fungal propagation, followed by frequent infections in living embryos during large-scale production.
After hatching, the development of yolk-bearing prolarvae, up to the evacuation of the pigment plug, was also significantly stimulated under the higher incubation temperature (20℃ in this study). The timing window for the evacuation of the pigment plug was also narrower in the 20℃ treatment than in the other treatments. Transition to exogenous feeding in sturgeon larvae is highly correlated with the ejection of the pigment plug after yolk sac depletion. Larval viability and development are sensitive to the availability of exogenous food, which needs to be provided in a timely manner after the expulsion of the pigment plug (Conte et al., 1988; Gisbert and Ruban, 2003). For this reason, the uniformity in the transition from yolk sac nutrition to exogenous feeding would be beneficial for larval rearing in sturgeon hatcheries. Taken together, the results of this study strongly suggest that an incubation temperature close to 20℃ is beneficial for both embryonic development and prelarval incubation in Siberian sturgeon, as evidenced by the shortened period and synchronized pattern. Further studies are needed to validate the present results and to determine if they can be replicated under various hatchery conditions. If such future studies are successful, the data from this study will provide a useful technical guideline for hatchery practices and the management of Siberian sturgeon.
Transient development of teeth in prolarvae and larvae of Siberian sturgeon Acipenser baerii during 5 days post hatching (dph) to 21 dph under the constant temperature condition (18 ℃). The teeth rudiments were firstly observable at 5 dph. Afterward, the development of teeth was peaked at 9 to 11 dph, and then began to degenerate. At 21 dph, teeth were completely diminished.


![Development of dorsal scutes Siberian sturgeon Acipenser baerii larvae during the phase of exogenous feeding (14 days post hatching [dph] to 20 dph). The 5th to 7th scutes from the dorsal fin were indicated by arrows. Scale bar = 5 mm.](http://oak.go.kr/repository/journal/12019/E1HKAL_2013_v16n1_25_f002.jpg)