Inflammation results from a host response to pathogenic challenges or tissue injuries, and ultimately leads to the restoration of normal tissue structure and function. Normal inflammatory responses are self-limited by a process that involves the down-regulation of pro-inflammatory proteins and the up-regulation of anti-inflammatory proteins (Lawrence et al., 2002). Thus, the physiological benefits of acute inflammation are limited, particularly in response to infectious pathogens, and chronic inflammation can lead to several inflammatory diseases (Kaplanski et al., 2003). Prolonged inflammation contributes to the pathogenesis of inflammatory diseases such as bronchitis, gastritis (Fichtner-Feigl et al., 2005), inflammatory bowel disease, multiple sclerosis (Klotz et al., 2005), and rheumatoid arthritis (Ponchel et al., 2002). Macrophages play an important role in a variety of disease processes, including autoimmune diseases, infection, and inflammatory disorders (Pierce, 1990).
Lipopolysaccharide (LPS), an endotoxin derived from the outer membrane of Gram-negative bacteria, can directly activate macrophages to produce a variety of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukins (ILs), and other inflammatory mediators such as prostaglandins and nitric oxide (NO) (Yoon et al., 2011). NO is endogenously produced from L-arginine and molecular oxygen by NO synthases (NOSs). In mammals, there are three isoforms of NOS: neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). nNOS and eNOS are constituvely expressed in neuronal and endothelial cells, respectively. In contrast, iNOS is inducible and its expression is increased in cells that are exposed to LPS or cytokines (Liu et al., 1998). Accordingly, high expression levels and activity of iNOS are observed in patients with chronic diseases such as inflammation and cancer (Liu et al., 1998; Maeda and Akaike, 1998).
Marine algae have been identified as rich sources of structurally diverse bioactive compounds with great pharmaceutical potential (Abad et al., 2008; Blunt et al., 2010). A variety of biological compounds including phlorotannins and fucoxanthin, have been isolated from Laminariacceae and characterized with regard to biological activity (Okuzumi et al., 1993; Kim et al., 2005; Woo et al., 2009). Sea tangle,
Sea tangle was added to water at a ratio of 1:15 (w/v), and 2% (w/w of dry sea tangle) rice flour was added to aid fermentation. After autoclaving at 121℃ for 30 min, a
>
Cell culture and cell viability assay
RAW 264.7 cells were grown to confluence in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum at 37℃ in a humidified atmosphere of 5% CO2. The cytotoxicity of the various components of the FST fractions was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, a method based on MTT (Weislow et al., 1989). The medium containing RAW 264.7 cells was cultured in a 96-well plate at a density of 105 cells per mL. The plate was incubated overnight and treated with 100 μL of DMEM medium containing different concentrations on FST. After 24 h of incubation, an MTT solution was added to each well and the plate was incubated for another 4 h at 37℃. The blue formazan salt was dissolved in dimethyl sulfoxide. Optical density was measured at 540 nm with a GENios microplate reader (Tecan, Austria GmbH, Grodig, Austria). The optical density of formazan formed by untreated cells was taken as the measure of 100% viability.
RAW 264.7 cells (1 × 106) were plated and incubated for 24 h with 0-100 μg per mL FST in the absence or presence of LPS (1 μg/mL). After treatment with LPS and FSTs, the nitrite concentration in the culture medium was measured as an indicator of NO production, according to the Griess reaction (Kim et al., 1995). For this measurement, 100 μL of culture supernatant was mixed with the same volume of Griess reagent (0.1% naphtylethylenediamine dihydrochloride and 1% sulfanilamide in 5% H3PO4), and the absorbance of the mixture was measured at 540nm with a microplate reader (Ultraspec 2100 Pro; Amersham Biosciences, Buckinghamshire, UK). The concentration of nitrite was calculated using sodium nitrite as a standard.
>
RNA extraction and reverse transcription-polymerase chain reaction
Total RNA was isolated using a Trizol reagent (Invitrogen Co., Carlsbad, CA, USA) following the manufacturer’s recommendations. Total RNA was digested with RNase-free DNase (Roche, Indianapolis, IN, USA) for 15 min at 37℃ and repurified using an RNeasy kit according to the manufacturer’s protocol (Quiagen, Valencia, CA, USA). cDNA was synthesized in accordance with the manufaturer’s instruction. Briefly, 2 μg of total RNA were incubated at 37℃ for 1 h with AMV reverse transcriptase (Amersham Biosciences) and random hexanucleotides. The primers used to specifically amplify the genes of interest are given in Table 1. Amplification was performed in a master-cycler (Eppendorf, Hamburg, Germany) with denaturation at 95℃ for 30 s, annealing at 60℃ for 45 s, and extension at 72℃ for 1 min. The amplified polymerase chain reaction (PCR) products were separated in 1.0% agarose gels and visualized by staining with ethidium bromide (EtBr).
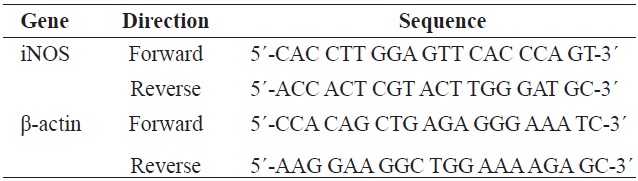
[Table 1.] Gene-specific primers used for the reverse transcription PCR
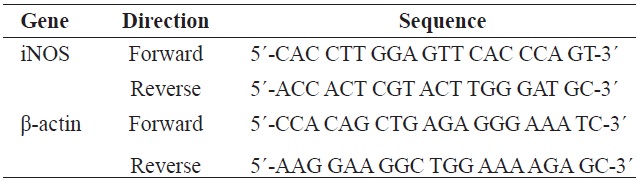
Gene-specific primers used for the reverse transcription PCR
Western blotting was performed according to standard procedures. Briefly, cells were lysed at 4℃ for 30 min in RIPA buffer containing 50 mM Tris-HCl (pH 8.0), 0.4% Nonidet P-40, 120 mM NaCl, 1.5 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride, leupeptin (80 μg/mL), 3 mM NaF and 1 mM dithiothreitol. Cell lysates (50 μg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene fluoride membrane (Amersham Pharmacia Biotech., Buckinghamshire, UK), blocked with 5% skim milk, and hybridized with primary antibodies (diluted 1:1000). After incubation with horseradish-peroxidase- conjugated secondary antibodies at room temperature, immunoreactive proteins were detected using a chemiluminescent ECL assay kit (Amersham Biosciences) according to the manufacturer’s instructions. Western blot bands were visualized using a LAS3000 Luminescent image analyzer (Fujifilm Life Science, Tokyo, Japan).
>
Effects of FSTs on RAW 264.7 cell viability
Inflammation is the first response of the immune system to infection or irritation. During inflammation, macrophages provide a host defense against extracellular foreign agents and stimuli. LPS is one of the major inflammatory stimuli that can induce the production of a variety of pro-inflammatory cytokines, including TNF-α, IL-1, and IL-6 in macrophages. Thus, inhibitors of these cytokines have been considered as candidate for anti-inflammatory drugs (Yoon et al., 2011).
In East Asia, sea tangle is consumed in a variety of ways: as
a side dish, in soups and salads, and as a sandwich ingredient. Traditionally, sea tangle has been used to lessen inflammation in oriental medicine. However, few studies have directly and systematically evaluated the effects of sea tangle extract on inflammation. This study examines the anti-inflammatory activity of FST using LPS-induced Raw 264.7 cells.
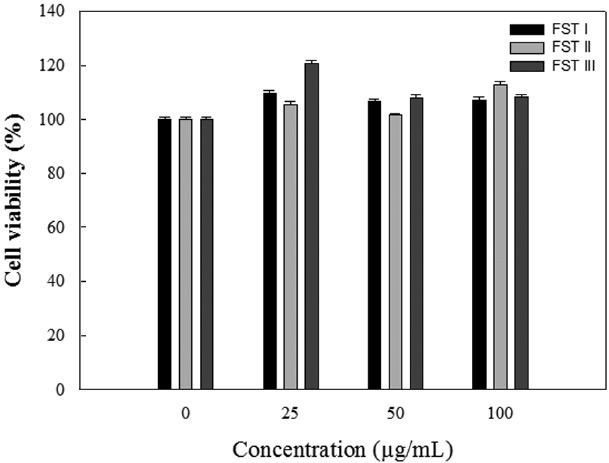
The cytotoxicity of FSTs on LPS-induced RAW 264.7 cells was determined using MTT assays. LPS-induced RAW 264.7 cells were treated with FSTs (0, 25, 50 or 100 μg/mL). As shown in Fig. 1, the FSTs did not show any significant cytotoxicity at concentrations less than 100 μg/mL. Thus, FSTs were deemed safe for
>
Effects of FSTs on NO production in RAW 264.7 cells
NO, an important cellular secondary messenger is a free radical that is produced at the cellular level from its precursor ?-arginine by NOSs. Several beneficial biological activities have been attributed to NO, including antimicrobicidal, antiviral, immunomodulatory, and antitumoral effects. However, high levels of NO are involved in the physiology and pathophsiology of several human diseases, and its uncontrolled release can cause destruction of target tissues during the inflammation processes (Yoon et al., 2011).
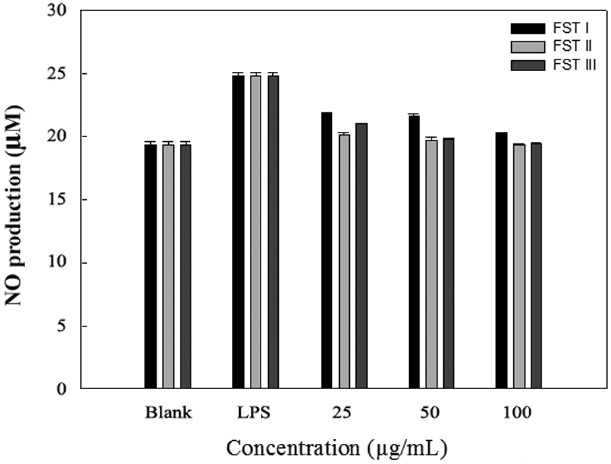
LPS can induce the formation of iNOS and NO in macrophage cells. Therefore, we investigated the inhibition of NO production by FSTs in LPS-induced RAW 264.7 cells. As an indicator of NO production, nitrite (NO2 ?) accumulation was measured in the culture media using the Griess method (Yoon et al., 2011). Fig. 2 shows that the FSTs inhibit LPS-induced NO production in macrophages. The inhibition may be due to
the suppression of LPS-induced iNOS transcription. Song et al. (2011) previously reported that
>
Effects of FSTs on the regulation of inflammatory response genes and proteins
FST treatment of LPS-activated RAW 264.7 macrophages resulted in a decrease in NO production. Nuclear factor-κB is critical to the regulation of cell survival genes and the coordination of pro-inflammatory mediators such as iNOS and NO (Kang et al., 2011). Therefore, the modulation of iNOS expression by sea tangle extract will impact transcription complex activity. Several studies have shown an elevation of iNOS levels in LPS-induced acute inflammatory responses. Since the modulation of iNOS-mediated NO production is one of the major contributing factors in the inflammatory process, the selective inhibition of iNOS may be beneficial for the treatment of inflammatory disease (Song et al., 2011).
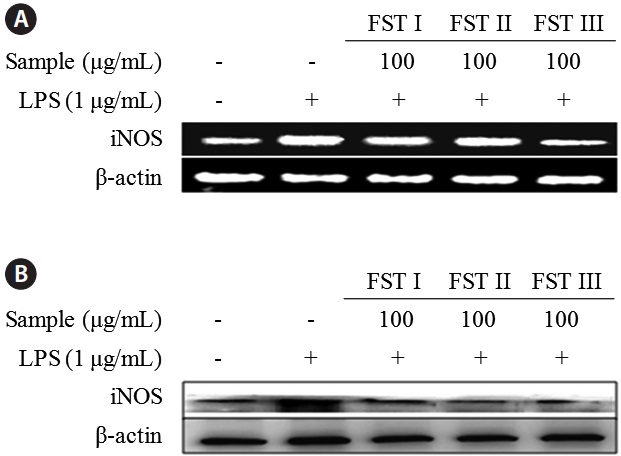
The effects of FSTs on cytokine mRNA levels and protein expression in LPS-induced RAW 264.7 cells were examined using reverse transcription PCR and Western blot analyses, respectively (Fig. 3). Levels of both mRNA and iNOS were reduced by FST treatment (at 100 μg/mL), which is consistent with the results obtained above showing an FST-related inhibition of NO production. This indicates that FSTs are an effective inhibitor of LPS-stimulated iNOS expression and NO secretion in RAW 264.7 macrophages. These results are consistent with those obtained with
In conclusion, the present study demonstrates that the anti-inflammatory effects of FSTs are the result of reductions in the levels of pro-inflammatory cytokines and the inhibition of iNOS expression and NO release in LPS-stimulated RAW 264.7 macrophages. Thus, FSTs may be used as a therapeutic agent for inflammatory diseases without side effects.