Generally, variations in hematological parameters are related to the fish species, age, sexual maturity, and health conditions (Blaxhall, 1972; Hrubec et al., 2000; Jamalzadeh et al., 2009). Normal ranges for various hematological parameters in fish have been established by different studies of fish physiology and pathology (Blaxhall, 1972; Ram Bhaskar and Srinivasa Rao, 1989; Hrubec et al., 2000; Martins et al., 2008). Hematological parameters can be affected by bacterial infection (Martins et al., 2008), parasitic infection (Ghiraldelli et al., 2006), and poor water quality (Ram Bhaskar and Srinivasa Rao, 1989). Hematological tests and the analysis of serum constituents provide crucial information for monitoring the health of fish (Aldrin et al., 1982). Specifically, these approaches provide reliable information on metabolic disorders, deficiencies, and health status before they become disease problems in cultivated fish. Regular monitoring of the hematological parameters of farmed fish can prevent losses due to fish diseases in aquaculture (Ram Bhaskar and Srinivasa Rao, 1989). Currently, there is no information available regarding hematological changes in olive flounder following
Two strains of
Normal olive flounder, with an average body weight of 47.28 g and body length of 17.01 cm, were obtained from a farm in Geoje Island and maintained in an aquaria at approximately 30 practical salinity units (psu), 18-20℃ and pH 8.0 (±0.2) for two weeks prior to the experiment. The fish were fed with commercial fish pellets (National Federation of Fisheries Cooperatives Feed, Korea) until they were used for the experiment.
Pathogenicity of these two strains was determined
>
Hematological study of olive flounder following V. scophthalmi infection
Hematological examination
The fish were sacrificed with a lethal dose of benzocaine anesthetic (CAS, Canada). Fish blood was drawn from the caudal vein with a syringe treated with an anticoagulant. Samples were then centrifuged at 300
Biochemical examination
Peripheral blood was drawn with a syringe without anticoagulant and allowed to clot for 1 h at room temperature and 2 h at 4℃. After centrifugation at 300
>
Survival of V. scophthalmi in fish serum and skin mucus
Serum and skin mucus of olive flounder were prepared following the techniques described by Bordas et al. (1996). The bacterial cells were suspended in fresh serum or skin mucus (1:1, v:v) and adjusted to 1 × 106 CFU/mL bacterial cells. One hundred microlitres of each sample was removed at 0, 1, 3, 6, and 18 h after incubation at 25℃ and 10-fold serial dilutions of PBS were spread on ST plates. The survival rate of strains in serum or skin mucus was defined as the number of viable bacteria after the co-culture was divided by an initial bacterial count as described by Leung et al. (1994).
>
Intracellular survival of V. scophthalmi in macrophages in vitro
Macrophage monolayer preparation
Monolayers of head kidney macrophages from olive flounder individuals were prepared as described by Secombes (1990) with slight modifications. The head kidney was removed, ground, and filtered through a sterile nylon mesh with L-15 medium (Gibco, Grand Island, NY, USA) containing 2% fetal bovine serum (FBS, Gibco), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10 U/mL heparin (Sigma). The cell suspension was layered onto a 34-51% Percoll density gradient in siliconized tubes on ice and centrifuged at 400
Intracellular survival of V. scophthalmi in macrophages
All values for the hematological and biochemical parameters were expressed as a mean ± standard deviation (SD). Hematological parameters in fish of different treatment groups were analyzed by analysis of variance (ANOVA) using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Clinical signs in olive flounder were similar between fish infected both experimentally and naturally and included darkening skin, distended abdomen, hemorrhages of muscles, liver, and intestine, ascites, and hypertrophy of the spleen and kidney. The inoculated strains could be reisolated from the liver, spleen, kidney, and ascites of dead individuals.
>
Hematological alterations of the olive flounder following V. scophthalmi infection
Hematological analysis
The effects of
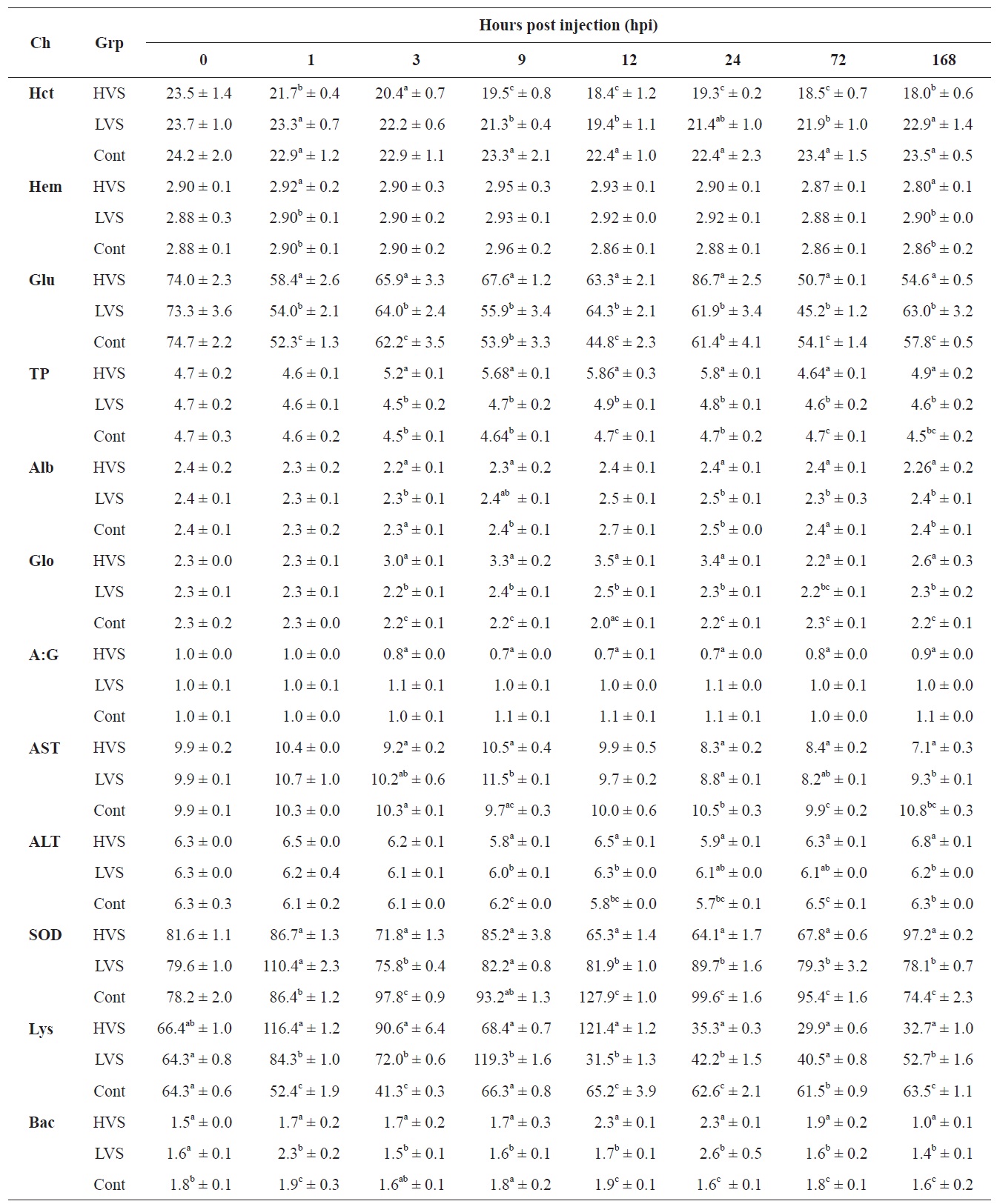
Hematological changes in olive flounder Paralichthys olivaceus following infection by different strains of Vibrio scophthalmi
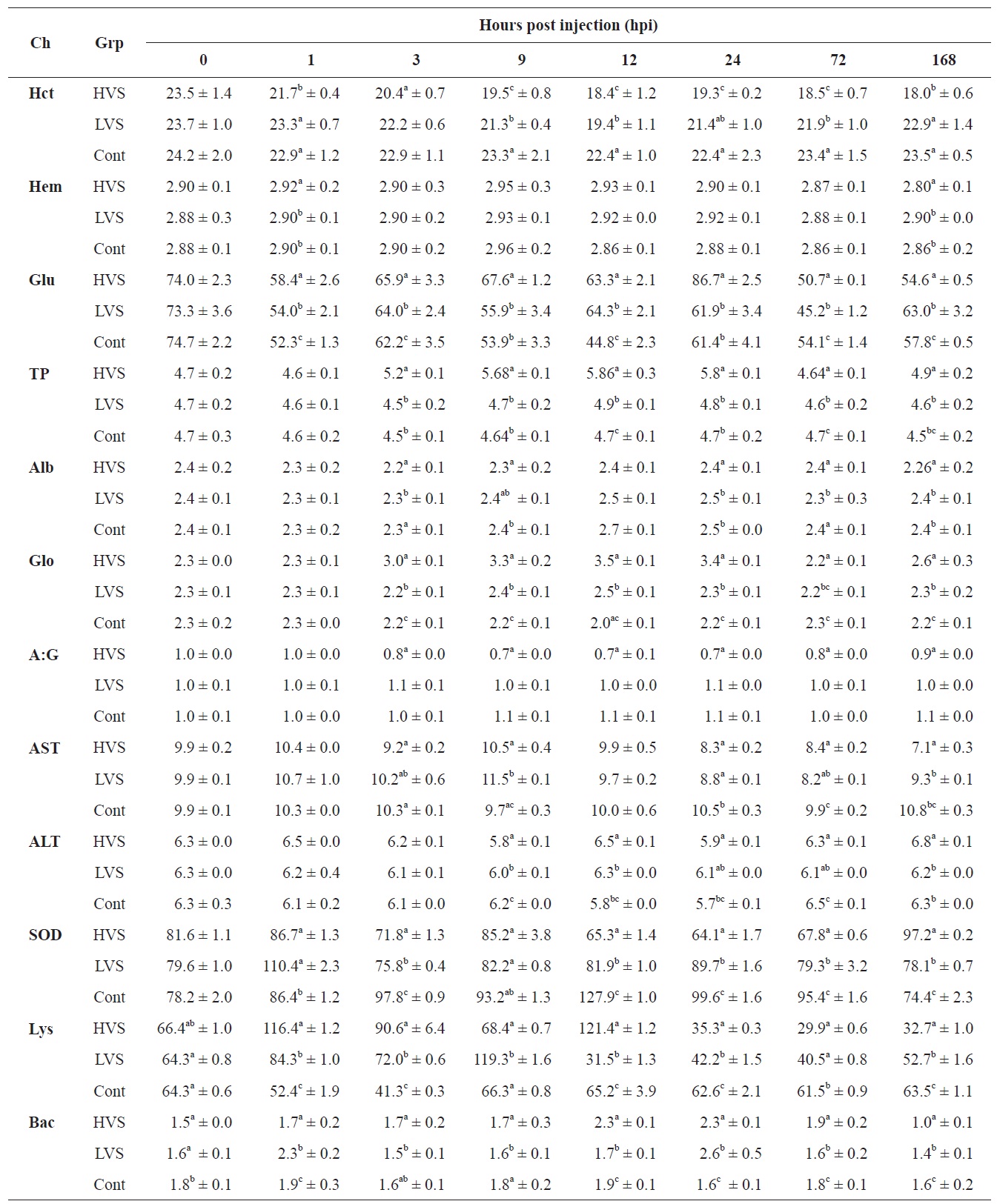
compared to fish in the control group. In fish infected with the HVS, the Hct value declined to 18.0% compared to the control group (23.5%) at 168 hpi. The Hb concentration increased to 2.95 g/dL at 9 hpi, and decreased to 2.80 g/dL at 168 hpi which was similar to the value at 0 h.
Biochemical examination
Serum biochemical values are shown in Table 1. The glucose concentration decreased significantly in the HVS and LVS infection groups compared to the control group from 1 to 24 hpi. TP concentration in the HVS infection group was significantly higher than in the LVS and control groups from 3 to 24 hpi. TP concentration increased to 5.86 g/dL at 12 hpi, as compared with 4.7 g/dL that in the control fish. The A:G ratio in the HVS infection group decreased significantly after 3 hpi and was significantly lower when compared to the control fish over the period from 3 to 168 hpi. Fish infected with the HVS had an A:G ratio of 0.69 at 12 hpi, significantly lower than the ratio of 1.03 A:G recorded in the control fish. The AST value and SOD activity in fish infected with the HVS also declined significantly after 12 hpi compared to those in the control group. ALT activity was not significantly different between infected and control groups. Lysozyme activity was significantly different between infected and control groups. Lysozyme activity in the HVS and LVS infected groups was
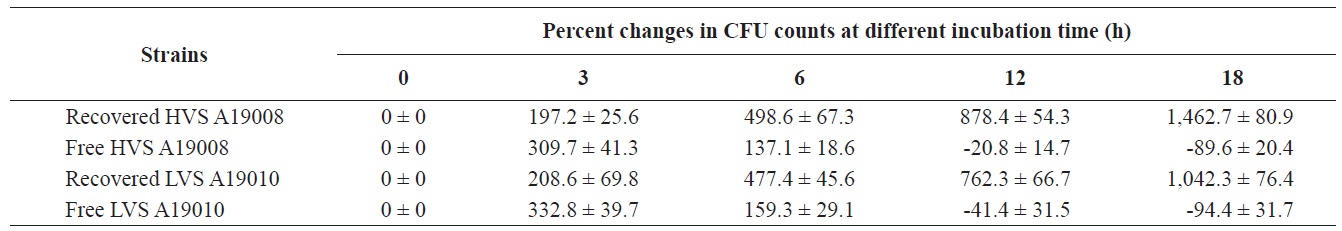
Intracellular survival of Vibrio scophthalmi in olive flounder Paralichthys olivaceus macrophages in vitro
enhanced significantly from 1 to 12 hpi and then decreased from 24 to 168 hpi compared to the control fish.
Bactericidal activity of serum
As shown in Table 1, bactericidal activity of serum in the HVS and LVS infected groups increased over time post infection and reached the highest values (2.3-2.6) at 24 hpi, which was significantly higher than the corresponding value in the control fish (1.6).
>
Survival of V. scophthalmi in fish serum and skin mucus
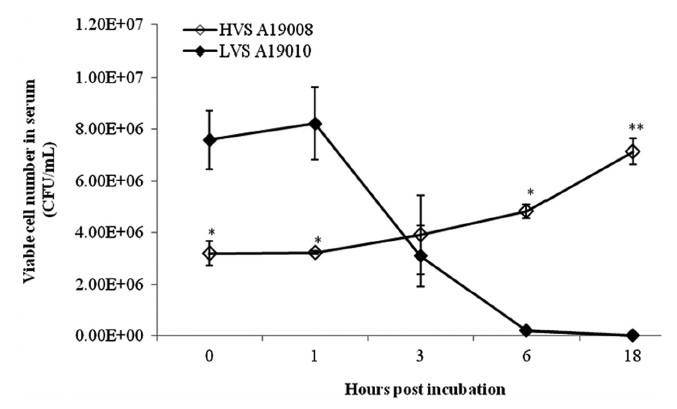
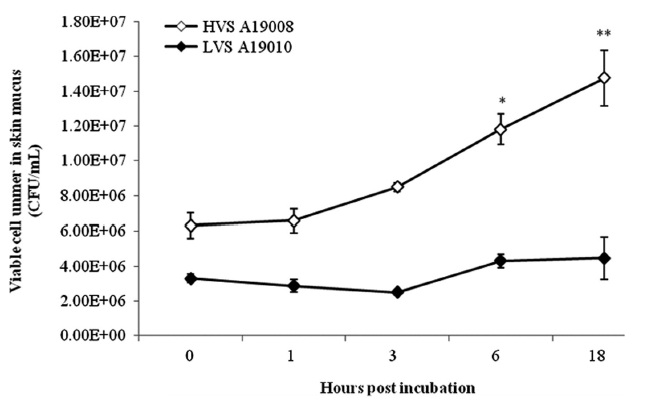
The HVS could survive in fresh serum and cell numbers increased by approximately 2 log units at 18 hpi (Fig. 1). The LVS showed serum sensitivity and cell numbers decreased below the detection limit. The HVS was able to survive and replicate in skin mucus with cell numbers increasing from 7.33 × 105 to 1.59 × 106 CFU/mL, whereas the LVS maintained cell numbers at the original levels in skin mucus (Fig. 2).
>
Intracellular survival of V. scophthalmi in macrophages in vitro
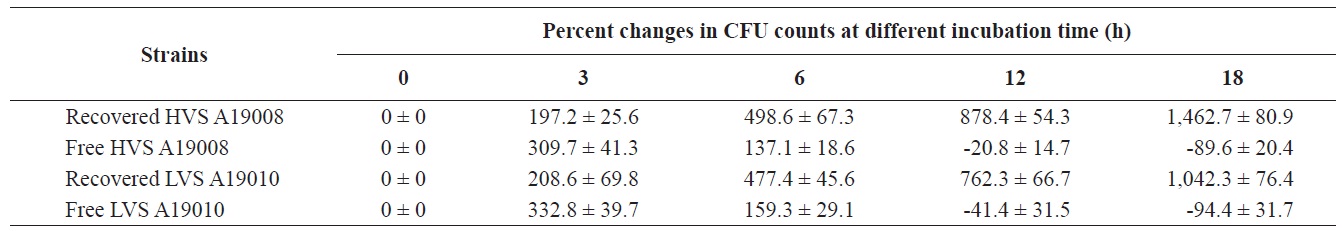
As shown in Table 2, the HVS was internalized in macrophages and cell numbers increased significantly from 0% to 197.2%, 498.6%, 878.6%, and 1,462.7% after incubation for 3, 6, 12, and 18 h, respectively, despite the presence of norfloxacin in the media. In contrast, cell numbers in media without macrophages, but with norfloxacin, declined by 20.8% and 89.6% at 12 and 18 h, respectively, when compared to cells at 0 h. For the LVS, cell numbers increased by 208.6%, 477.4%, 762.3%, and 1042.3% after incubation for 3, 6, 12, and 18 h, respectively. Cell numbers of the LVS in medium with norfloxacin without macrophages declined 41.4% and 94.4% at 12 and 18 h, respectively, when compared to time 0 h. The HVS could survive and multiply in macrophages better than the LVS.
Hematological characteristics are important for fish and can be used as an effective and sensitive index to monitor physiological and pathological conditions (Kori-Siakpere et al., 2005). In this study, TP concentration in the serum of olive flounder in the infected groups was significantly higher than that of control fish, which was consistent with observations made by Lee (2005). Lee (2005) found that TP concentration in fish injected with different concentrations of ECP extracted from
AST and ALT are jointly known as transaminases and are associated with inflammation or liver injury. AST is also found in many other organs in addition to the liver, including kidneys, muscles, and the heart. ALT is found primarily in the liver and high levels of ALT are almost always indicative of liver problems. In this study, ALT was observed to increase, indicating liver damage. Moreover, the ALT:AST ratio may also provide useful information regarding the extent and cause of liver disease, because most liver diseases are characterized by greater elevations of ALT than AST.
Lysozyme is a cationic enzyme that breaks down β-1, 4 glycosidic acids and N-acetyl-glucosamine in the peptidoglucan of bacterial cell walls. It plays an important role in the bio-defense system against Gram-positive and Gram-negative bacteria (Alexander and Ingram, 1992). In this study, there were significant changes in the lysozyme activity of infected fish compared to the control fish. The infected fish were significantly stimulated before 12 hpi and lysozyme activity reached a high level within a short period of time after infection. The bactericidal activity in infected fish also reached a high level before 72 hpi, suggesting that lysozyme levels could be correlated with phagocytic activity. The bactericidal activity of serum has been well recognized as one of the key mechanisms for clearing bacteria from fish (Ellis, 2001). In this study, the bactericidal activity in infected fish could not be maintained at a high level near the end of the experiment (168 hpi), probably due to the reduction in lysozyme secretion. Robertsen et al. (1990) demonstrated an enhanced protection against fish bacterial infection and confirmed a correlation with an increase in serum lysozyme, phagocytic, and bactericidal activity in head kidney phagocytes.
The HVS was able to survive and replicate in the serum of olive flounder individuals, whereas the LVS was unable to survive. This suggests that the survival and reproduction of
The results regarding the number of intracellular viable cells of bacterial strains revealed that both the HVS and LVS were capable of invasion and replication within macrophages
In summary, this study evaluated hematological and biochemical alterations for olive flounder infected by