The Antarctic krill
Despite its huge biomass, Antarctic krill has not been a traditional food in the human diet because of its high fluorine content. While small amounts of fluoride in food have been shown to help prevent tooth decay, too much fluoride in the diet or long-term excessive intake of fluoride can result in macular teeth (Arthur, 1971).
Both
Several reports describing the removal and/or quantitation of fluoride in krill and other foods have been published. A simple, rapid, and reliable analytical procedure for determining fluoride levels in biological samples employs alkali fusion and fluoride ion-selective electrodes (Kumagai, 1984; Malde et al., 2001). Kim et al. (1990) studied the migration of fluoride from chitinous components into the muscle of frozen krill (FK) during thawing and reviewed an electrocondensation method as a means of reducing the fluoride content of Antarctic krill. Fluorine removal during the production of krill paste and krill protein concentrates was studied by Filho (1993). The amount of soluble protein in krill muscle depends on pH, ion species, and the ionic strength of the extracting solutions (Park et al., 1988).
This report details the removal of fluoride from Antarctic krill using an optimized organic acid extraction. Such a pretreatment would allow krill to be used as a protein substitute in human food and aquacultural fish meal.
Antarctic krill
>
Measuring fluoride levels by alkali fusion
The fluoride content of the krill was determined using a method developed by Malde et al. (2001). Approximately 0.25-0.50 g samples were weighed directly into nickel crucibles. The samples were covered with 2.5-5.0 mL of 8 M sodium hydroxide, depending on the amount of sample. The crucibles were put on a hot plate and evaporated to dryness before being put into a muffle furnace for combustion. The muffle furnace temperature was set at 200℃ for approximately 16 h and then raised to 525℃ and held for another 3 h. The crucibles were cooled, 10-15 mL of distilled water was added, and the crucibles were placed on a hotplate to aid the dissolution of the fusion cake. After approximately 2 h, the sample solutions were transferred to 50 mL plastic tubes and capped.
The sample solutions were then neutralized with concentrated hydrochloric acid, added dropwise until the pH decreased from 12.0-13.0 to 8.0-8.5. The pH was then reduced to 7.2-7.5 by adding dilute hydrochloric acid with a titrator. The sample solution was transferred to a 50-mL plastic volumetric flask and stored until analysis. Aliquots of 10 mL were removed from the volumetric flask and 1 mL of TISAB III was added to each aliquot, bringing the pH to 5.2-5.4. Reagent blanks were used as negative controls and standard solutions were prepared with fluoride concentrations of 0.100, 1.00, and 10.0 mg F?/L. The standard and sample solutions were analyzed with a fluoride ion-selective electrode (9609BNWP and 960900 fluoride combination electrode, Orion Dual Star, pH/ISE Benchtop; Thermo Scientific, Waltham, MA, USA). The electrode was rinsed and blotted dry before each measurement.
>
Measuring fluoride levels by organic acid extraction
Aliquots of 0.5-2.0 g of lyophilized FK were weighed into plastic beakers. To each aliquot was added 50-200 mL of 0.01-0.1 M citric acid and the mixtures were stirred with an agitator for 5-30 min. The mixtures were then centrifuged, filtered, and adjusted to pH 7.2-7.5 with a NaOH solution. Five milliliters of each filtrate was placed into a 10-mL volumetric flask. One milliliter of a TISAB III solution was added and the fluoride concentration was measured using the fluoride ion-selective electrode described above.
>
Percent recovery using fluoride standard solutions
To a beaker were added 0.5 g samples of FK and KM, an aliquot of fluoride standard solution, and 50 mL of 0.01 M citric acid. The mixture was then stirred for 5 min and filtered. Tenmilliliter aliquots of filtrate were removed from the beaker, adjusted to pH to 7.2-7.5 with 0.1 M NaOH, and transferred to a 10-mL volumetric flask. One milliliter TISAB III was added the flask and the fluoride concentration was measured with an ion-selective electrode as above. Percent recovery test was calculated as follows:
Percent recovery of added fluoride = (F2 - F1)/F3 × 100%
F1: Fluoride content of the sample
F2: Fluoride content of the sample after adding the fluoride standard
F3: Amount of added fluoride
All of the measurements performed on powders of KB, KM, and BK were done in triplicate. The results of each measurement are expressed as the mean ± SD. Analysis of variance (ANOVA) and Duncan’s multiple range tests were used to determine statistical significance at
>
Type and concentration of organic acids
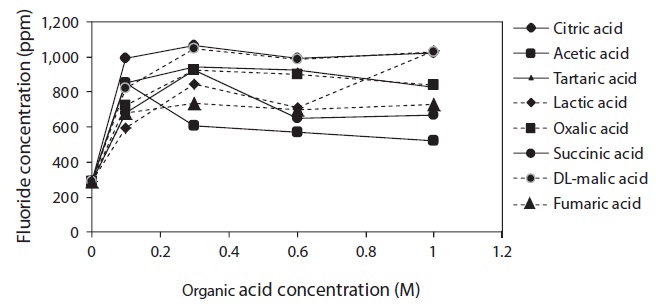
Fig. 1 shows the changes in fluoride concentration of solutions extracted from lyophilized FK samples as a function of acid concentration. Citric, acetic, tartaric, lactic, oxalic, succinic, malic, and fumaric acids were evaluated for their ability to remove fluoride from lyophilized FK.
The degree of fluoride removal increased significantly with increasing organic acid concentration from 0.001 to 0.1 M. Citric acid was particularly effective at extracting fluorine from lyophilized FK, although malic, tartaric, and lactic acids were also effective at a concentration of 1.0 M. Above 0.1 M citric acid, however, the amount of extracted fluoride remained the same. Most of the organic acids exhibited their highest extraction efficiency at concentrations between 0.1 and 0.3 M. Succinic, lactic, oxalic, and fumaric acids exhibited steadily increasing efficiencies from 0.1 to 1.0 M, but yielded unstable extraction efficiencies at higher concentrations. Among the eight organic acids, citric acid was the most effective in extracting fluoride from lyophilized FK.
>
Extraction time and temperature
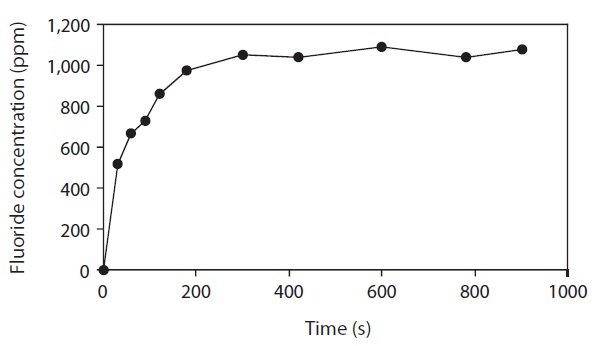
The amount of fluoride extracted from FK increased with increasing extraction time until reaching a plateau at 300 s (Fig. 2).
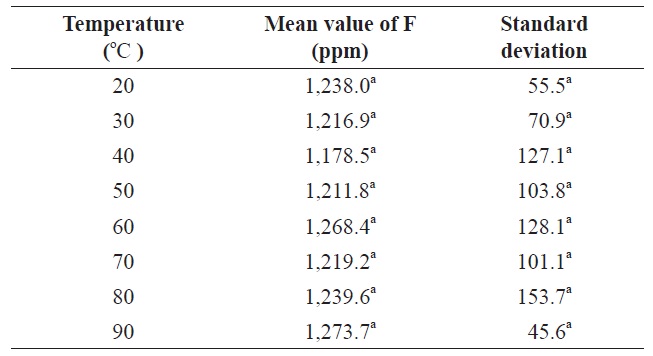
Therefore, a 5-min extraction time was deemed sufficient with 0.1 M citric acid solution. Kumagai (1984) reported that the extraction of fluoride from krill using 0.05 N HClO4 at room temperature was constant over 30 min. The effects of temperature on fluoride extraction with citric acid were also evaluated from 20℃ to 90℃. No significant difference (
>
Concentration of citric acid in the extraction solution
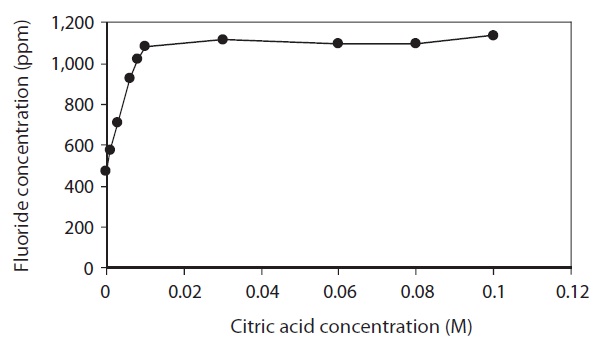
Lyophilized FK was treated with 0.001-0.10 M citric acid and the fluoride concentrations of the resulting extracts were measured (Fig. 3). With 1.0 g of FK and 50 mL of citric acid solution, fluoride levels in the extracted solutions increased with increasing citric acid concentration from 0.001 M until reaching a plateau at 0.01 M. Thus, the optimum concentration of citric acid for fluoride extraction was 0.01 M.
>
Volume of the extraction solution
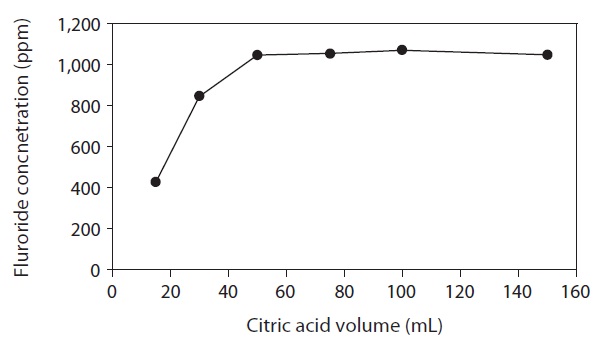
Fig. 4 shows the fluoride removed from lyophilized FK as a function of citric acid extraction solution volume from 15 to 150 mL. The extraction was performed for 5 min on a 1g sample. Extracted fluoride levels increased with the volume of the extraction solution from 15 to 50 mL. No further increase
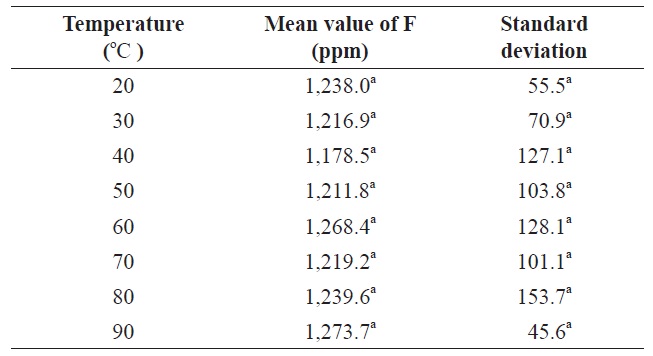
Effect of temperature of citric acid solution on fluoride contents in krill Euphausia superba
[Table 2.] Recoveries of fluoride from frozen krill and krill meat (dry basis)
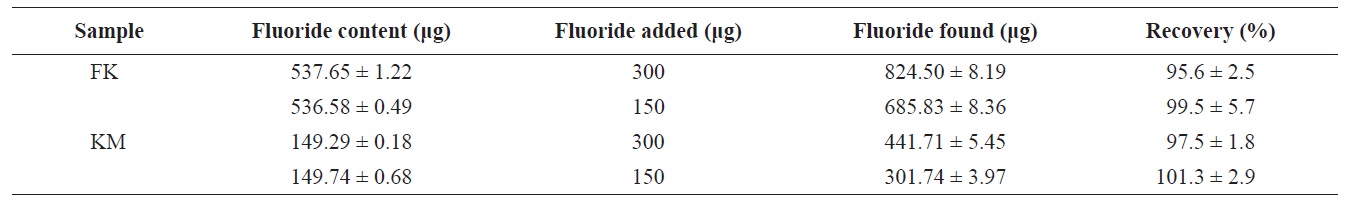
Recoveries of fluoride from frozen krill and krill meat (dry basis)
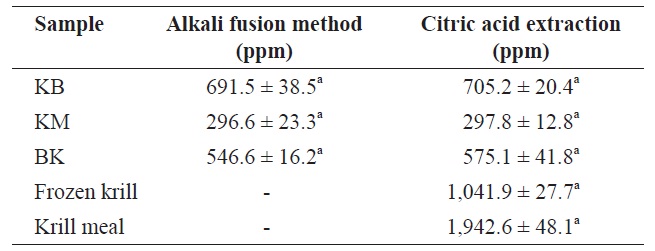
Comparisons of fluoride contents obtained by alkali fusion method and by citric acid extraction (dry basis)
was observed at volumes greater than 50 mL. Thus, the optimum volume of 0.01 M citric acid solution for the extraction of fluoride from lyophilized FK was 50 mL.
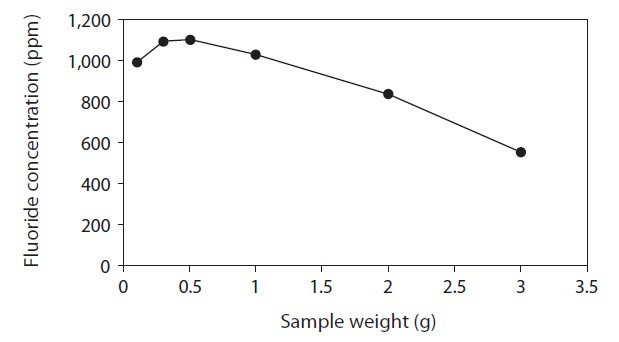
To optimize sample size for fluoride extraction, fluoride concentrations were measured after 5-min extractions (with 50 mL of 0.01 M citric acid) of 0.1-3.0 g lyophilized FK (Fig. 5). The amount of fluoride eluted increased from 0.1 to 0.5 g and then decreased with increasing sample sizes above 0.5 g (Fig. 5). Therefore, with extractions performed using the aforementioned optimized parameters 0.3-0.5 g of sample were ideal.
>
Percent recovery of fluoride from krill by standard additions
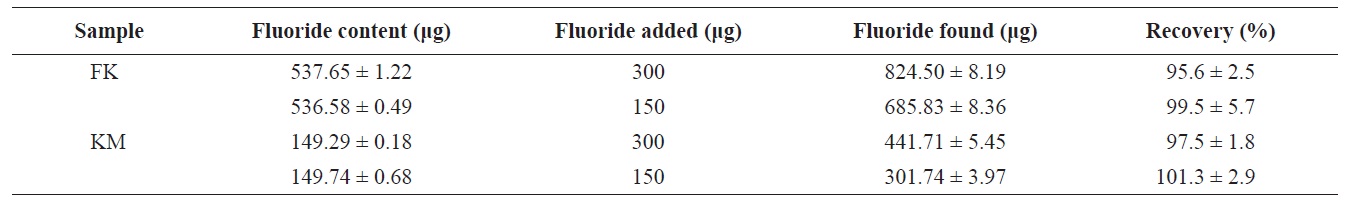
The percent recovery of fluoride (as determined by standard additions) from lyophilized FK and KM are given in Table 2. Recoveries of spiked fluoride (150 and 300 μg fluoride added to each sample) were 95.6 ± 4.7% and 99.5 ± 5.7% for lyophilized FK and 97.5 ± 1.8% and 101.3 ± 2.9% for lyophilized KM, respectively. Thus, recoveries were acceptable with a range of 95.6-101.3%. Kumagai (1984) reported recoveries of fluoride ranging from 93.3% to 96.9% for 18 samples of fluoride extract using an ion-selective electrode. Their extraction procedure consisted of shaking 1-2 g of sample in 100 mL of 0.05 N HClO4 for 45-60 min. Malde et al. (2001) reported fluoride recoveries from 80% and 110%. These data agree with the values obtained in the current study, although the present study used a lower ionic strength of citric acid and a shorter extraction time. No significant differences were observed in percent recovery between the samples and spiked fluoride solutions used in the present study.
>
Comparison of fluoride content as determined by alkali fusion methods and citric acid extraction
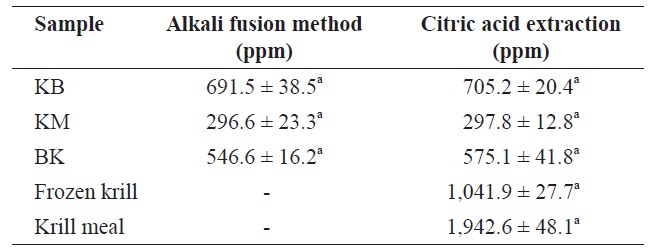
The fluoride contents of lyophilized FK, KM, and BK determined by alkali fusion methods and by citric acid extraction are given in Table 3. Levels of 691.46 ± 38.48, 296.58 ± 23.30, and 546.61 ± 16.23 ppm (dry matter), respectively, were obtained using alkali fusion methodsf 705.23 ± 20.38, 297.78 ± 12.77, and 575.11 ± 41.76 ppm (dry matter) were obtained using citric acid extraction for FK, KM, and BK, respectively. The two methods yielded statistically identical results (
Fluorine levels in frozen krill and krill meal were 1041.9 ± 27.7 and 1942.6 ± 48.1 ppm (dry matter), respectively. These high fluorine levels have been associated with the ability of krill to live in below-freezing waters (Soevik and Braekkan, 1979). For
The amount of soluble protein in krill muscle depends on the pH. The amount of extracted fluorine depends on the ionic strength of, and the types of ionic species in, the extracting solution. The amount of fluoride extracted from krill decreased from 64.5 to 8 ppm when extracting with an ionic strength of 0.05 M NaCl at pH 11 (Park et al., 1988). An electrocondensation method using an aluminum electrode was also developed as a means of removing excess fluoride from Antarctic krill. For the chitinous components of Antarctic krill, 30-70% of the total fluoride could be removed after 120 min of electrocondensation (Kim et al., 1990).
The results of the current study show that citric acid extraction was just as effective as the methods of Park et al. (1988) and Kim et al. (1990) for reducing the amount of fluoride in prepared krill samples. Citric acid is a common food additive that is considered safe for consumption throughout the food industry. In this study, the most effective extraction of fluoride from krill was achieved using 50 mL of 0.01 M citric acid for 5 min on 0.3-0.5 g samples.
Although present legislation in the Republic of Korea has set the maximum fluorine level for drinking water at 2.0 mg/L, 100 μg/g for fish protein concentrates is permitted by the US Food and Drug Administration (US FDA), and up to 2.5 and 4.0 mg of fluorine per day are considered safe dietary intakes for children and adults, respectively (National Academy of Sciences, 1989; Brown, 1990). A current need exists to determine acceptable limits of fluoride in various foods and to define maximum allowable levels of dietary fluoride prior to using krill biomass as a nutritional supplement for humans.