Pelvetia siliquosa (“Tumbugi” in Korean) belongs to the family Fucaceae. It is a marine alga endemic to the Korean peninsula and grows on the craggy surfaces of the southern seashores (Yoon, 1995). It has been used traditionally similarly to seasoned sea greens for religious services and as a health food (Oh et al., 1990).
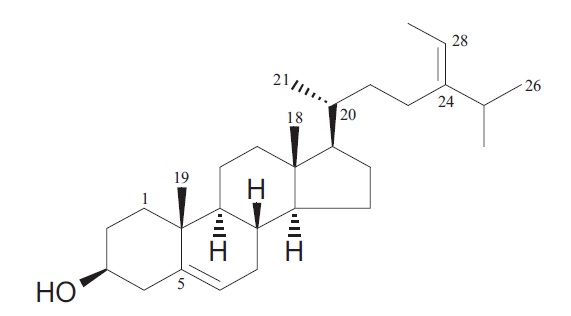
Results of our previous studies indicate that the ether fraction of P. siliquosa exhibits hepatoprotective and glucoselowering effects, and fucosterol isolated from P. siliquosa has antioxidant and antidiabetic activities (Lee et al., 2002, 2003, 2004b). Additionally, it has been reported that fucosterol decreases angiotensin-converting enzyme levels, with a reduction in the glucocorticoid receptor in endothelial cells and inhibits the lymphatic absorption of cholesterol in rats (Hagiwara et al., 1986; Ikeda et al., 1988). Thus, fucosterol is a major component responsible for the various activities of the algae. Fucosterol is usually isolated by extraction with a non-polar solvent and silica-gel column chromatography and is analyzed as the trimethylsilyl derivatives by gas chromatography. We have previously reported a method for the quantitative determination of fucosterol in marine algae to accurately determine their composition and thus generate fingerprints. The structural identification of fucosterol was performed with infrared spectrum (IR), electron impact-mass spectrum (EIMS), 1H NMR and 13C-NMR with tetramethylsilane (TMS) as internal standard. This structure was also confirmed by comparing with the previously reported spectral data. Its structure is shown in Fig. 1 (Lee et al., 2004a).
High-speed countercurrent chromatography (HSCCC) is a liquid-liquid partition chromatographic technique that has recently gained increased interest. This technique relies on the use of centrifugal force for the retention of the stationary phase. HSCCC is more advantageous than other liquid-liquid techniques because of the shorter separation time, wider range of possible solvent systems, and quantitative material recovery (Marston and Hostettmann, 1994, 2006; Ito, 2005; Friesen
and Pauli, 2007). These technical advantages have resulted in extensive use of HSCCC for the preparative separation of various natural products (Tsao and Deng, 2004; Wang et al., 2007).
In this study, we report for the first time one-step isolation and purification of fucosterol from a crude extract of P. siliquosa and rapid and efficient recovery of the constituents by HSCCC.
Pelvetia siliquosa Tseng et Chang (Fucaceae) was collected in the Jindo area, Jeonnam Province, in 2005 and was identified botanically by Professor Jong-Ahm Shin, Yosu National University, Korea. A voucher specimen was deposited at the Center for Efficacy Assessment and the Development of Functional Foods and Drugs, Hallym University, Chuncheon, Korea.
Methylene chloride, n-Heptane, acetonitrile, and methanol were purchased from Fisher Scientific (Schwerte, Germany)
for the preparation of crude extract and HSCCC separation. The sample was stored at -18℃ until required for experiments.
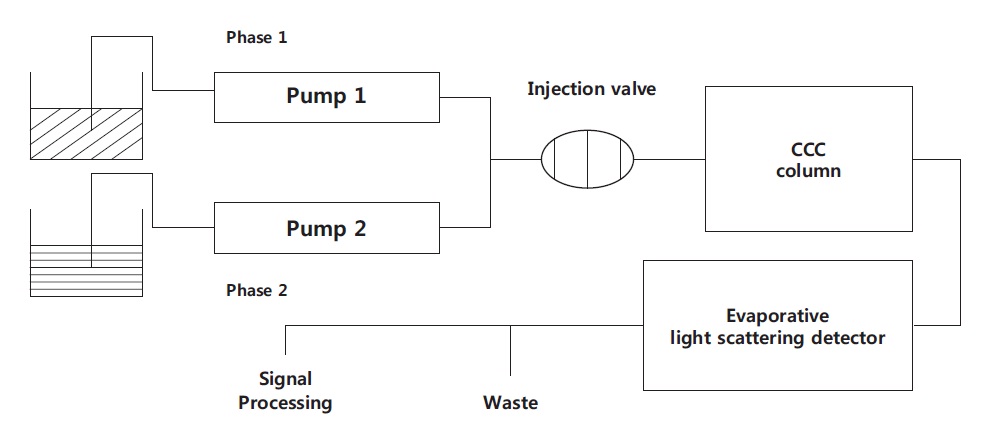
Preparative HSCCC was performed using a Model CCC- 1000 multilayer coil of polytetrafluoroethylene (PTFE) tubing with an inner diameter of 1.6 mm and a total capacity of 325 mL (Pharma-Tech Research, Baltimore, MD, USA). Considering a distance of r = 3.8-5.7 cm from the coil to the holder shaft and of R = 7.6 cm from the column axis to the central axis of the centrifuge, the β-value (β = r/R) varied from 0.50 (internal terminal) to 0.75 (external terminal). The revolution speed of the apparatus can be regulated with an electronic controller in the range 0-1,200 rpm. As illustrated in Fig. 2, the solvent was pumped into the column with a high performance liquid chromatography (HPLC) pump (Hitachi L-6200; Hitachi, Shimadzu, Japan), and the eluent was monitored continuously by connecting the tail outlet of the coiled column with an ELSD 2000ES through a flow-splitter valve assembly 200ES (Alltech Associates Inc., Bannockburn, IL, USA). A manual sample injection valve with a 10-mL loop was used to introduce the sample into the column. The data were also displayed and analyzed simultaneously on an Agilent HPLC workstation (Agilent Technologies, Boeblingen, Germany). IR data were recorded on a JASCO FT/IR?4100 spectrometer (JASCO, Tokyo, Japan). EI-MS conditions were: source temperature, 250℃; analyzer temperature, 120℃; electron energy, 70 eV.
Whole P. siliquosa was dried and then pulverized. The sample (100 g) was extracted twice with methylene chloride (1 L). The sonication conditions were 1.6 W/mL at 25℃ for 3 h, followed by rotary evaporation at 40℃ under reduced pressure, yielding 1 g of crude extract for HSCCC separation.
Selection of two-phase solvent systems
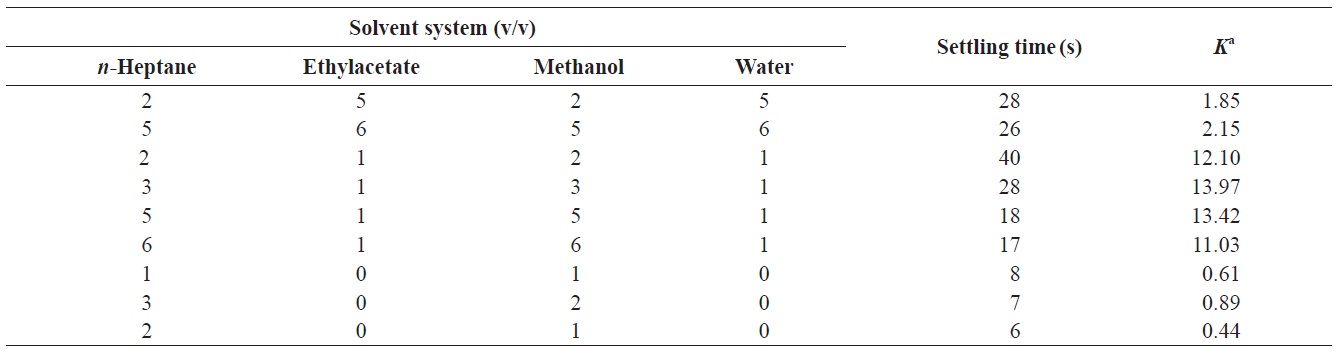
The composition of a two-phase solvent system was selected according to the partition coefficient (K) of the target compounds of the crude extract. Approximately 5 mg of the crude extract was weighted in a test tube, to which 1 mL of each phase of the pre-equilibrated two-phase solvent system was added. After the tube was shaken vigorously, the solution was separated briefly. The upper and the lower phases were then analyzed by HPLC to obtain the partition coefficient of the target compound. The K value was expressed as the peak area of target compound in the upper phase divided by that in the lower phase. The settling time, which is highly correlated with the retention in the stationary phase, was expressed as the time to form a clear layer between the two phases when the phases were mixed.
Preparation of two-phase solvent system and sample solution
The selected solvent system consisted of n-heptane:methanol= 3:2 (v/v) for HSCCC separation. Each component of the solvent system was added to a separating funnel and thoroughly equilibrated at room temperature. The two phases were separated and degassed before use.
HSCCC separation procedure
The multilayer coil column was first entirely filled with the upper organic phase (stationary phase) at a flow rate of 4.0 mL/min. The lower phase was then pumped into the head end of the inlet column at a flow rate of 1.2 mL/min, while the apparatus was run at 800 rpm. After hydrodynamic equilibrium was established, as indicated by elution of a clear mobile phase at the tail outlet, the sample solution (50 mg in 3 mL of each phase) was injected into the separation column through the injection valve. The effluent from the tail end of the column was monitored continuously by connection to the tail outlet of the coiled column with the evaporative light-scattering detection (ELSD) system through a split valve. The ELSD system was set to a probe temperature of 90℃ and a gain of 1.0, and the nebulizer gas nitrogen adjusted to 1.7 bars. Each peak fraction was collected according to the elution profile and assessed by HPLC. After separation was complete, retention of the stationary phase was measured by collecting the column contents; these were forced out of the column using pressurized nitrogen gas.
HPLC analyses and identification of fractioned peak
The crude extract and each peak fraction from HSCCC were analyzed by HPLC. The analyses were performed with an Agilent Eclipse XDB C18 column (150×4.6 mm I.D.) at a column temperature of 30℃. The mobile phase, consisting of acetonitrile-methanol (70:30, v/v) was isocratically eluted at a flow rate of 0.8 mL/min using the ELSD detector.
IR (KBr): 3,439, 2,936, 1,626, 823 cm-1. Mass (EI, 70 eV): m/z (%) = 412 [M]+ (119), 397 (3.8), 394 (2.8), 379 (3.7), 314 (100), 299 (23.9). 296 (16.7), 281 (24.5), 271 (12.6), 255 (5.6), 253 (5.1), 229 (21.4), 213 (11.5), 145 (10.7), 55 (25.3).
1H NMR (400 MHz, CDCl3): 5.36 (1H, br. d, J = 5.2 Hz, H-6), 5.19 (1H, q, J = 6.7 Hz, H?28), 3.53 (1H, m, H-3), 1.58 (3H, br s, H-21), 1.02 (3H, s, H-19), 1.01 (3H, br s, H-21), 1.00 (3H, d, J = 1.2 Hz, H-27), 0.98 (3H, d, J = 1.2 Hz, H-26), 0.70 (3H, s, H-18).
13C-NMR (100 MHz, CDCl3): 146.9 (C-24), 140.7 (C-5), 121.6 (C-6), 115.5 (C-28), 71.7 (C?3), 56.7 (C-14), 55.7 (C- 17), 50.1 (C-9), 42.3 (C-13), 42.2 (C-4), 39.7 (C-12), 37.2 (C-1), 36.5 (C-10), 36.4 (C-20), 35.2 (C-22), 34.7 (C-25), 31.8 (C-7,8), 31.5 (C-2), 28.2 (C-16), 25.6 (C-23), 24.3 (C- 15), 22.2 (C-26), 22.1 (C-27), 21.0 (C-11), 19.4 (C-19), 18.7 (C-21), 131.1 (C-29), 11.8 (C-18).
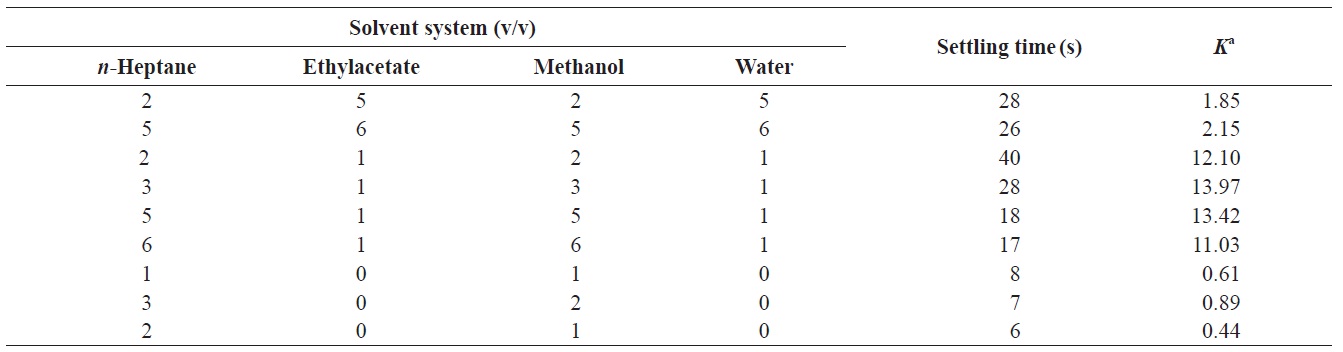
Successful separation by HSCCC depends largely on the selection of a suitable two-phase solvent system. This requires consideration of the following:the satisfactory retention of the stationary phase (Yoon, 1995), the settling time of the solvent system should be shorter than 20 s (Oh et al., 1990), the partition coefficient (K) should be close to 1 (Lee et al., 2002, 2003, 2004a), and the separation factor (α, α = K2/K1, K2 > K1) between the two compounds should be greater than 1.5. Generally, a small K value elutes the solute closer to the solvent front with lower resolution, while a larger K value tends to give better resolution; however, broader and more diluted peaks are obtained due to the longer elution time. Thus, suitable K values for HSCCC are 0.5 ≤ K ≤ 1.0 (Marston and Hostettmann, 1994, 2006; Ito, 2005; Friesen and Pauli, 2007).
To achieve efficient resolution of the target compound, the following two-phase solvent systems were examined: n-heptane- ethyl acetate-methanol-water (2:5:2:5, 5:6:5:6, 2:1:2:1, 3:1:3:1, 5:1:5:1, and 6:1:6:1 v/v/v/v) and n-heptane-methanol (1:1, 3:2, and 2:1, v/v). When the n-heptane-ethyl acetate/ methanol-water was used, the K value of fucosterol was large and it was eluted together with other compounds, resulting in poor resolution. When n-heptane-methanol was used, the K value and settling time of fucosterol were 0.89 and 7 s, respectively (Table 1), indicating an efficient resolution, short elution time, and good retention of the stationary volume during the separation.
In the present study, the retention of the stationary phase was 60.1%. The retention of the stationary phase relative to the total column capacity was measured from the volume of the stationary phase collected after complete separation. Our
results indicate that n-heptane-methanol at a ratio of 3:2 (v/v) was the most appropriate two-phase solvent system.
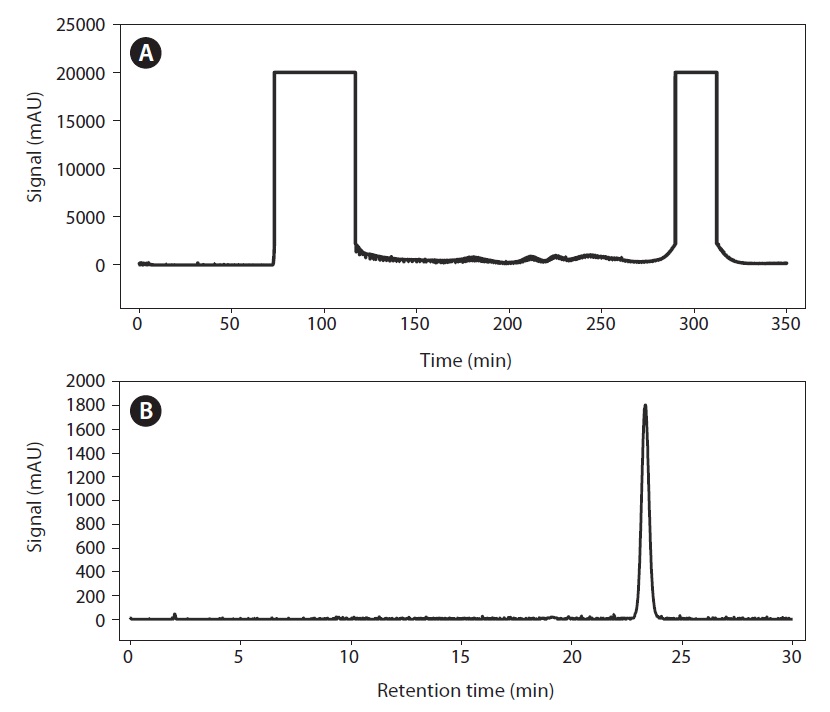
Fig. 3A shows the results of fucosterol separation by HSCCC from the crude P. siliquosa extract. The high-performance liquid chromatogram of the crude extraction, shown in Fig. 3B, revealed that the purity of fucosterol isolated by HSCCC was 96.8%. Additionally, the fucosterol content in the crude extract was 23.50%, calculated on the basis of HPLC peak-area percentage.
Thus, we developed an effective method for the preparative separation of fucosterol from P. siliquosa using HSCCC. One-step purification of fucosterol from 50 mg methylene chloride extract was achieved in 300 min. Our results indicate that HSCCC is a useful, rapid, and selective method for the separation of fucosterol from P. siliquosa.