



Thermosetting resins are polymer materials that irreversibly cure and generally come in liquid form. Curing may be carried out by applying heat or through a chemical reaction or irradiation with infrared light, ultraviolet light, or an electron beam. Once they have been cured, the resins cannot be reheated and melted back to their liquid form. Thermosetting resins are easy to process and laminate, and pressure or heat is not necessarily needed for their formation. In addition, they are generally inexpensive, stronger than thermoplastics, and better suited than thermoplastics to higher temperatures. However, they are more brittle than thermoplastics [1-5].
Thermoplastic resins consist of long polymer molecules that are generally not cross-linked. The resin can be repeatedly melted and reused. Usually, no chemical change occurs when the thermoplastic is cured. Thermoplastic resins are often supplied as granules and heated to permit fabrication by conventional molding methods, such as injection molding, rotational molding, extrusion, vacuum forming, and compression molding. Thermoplastic resins have high impact strength, and they are recyclable. Further, they give off no emissions, and they can bond to other thermoplastics and be molded or shaped with reheating.
Carbon fibers (CF) have become an important reinforcing material in advanced composites because of their extremely high strength, stiffness, and heat-resistance, and low weight. Fiber-reinforced polymer composites are of great interest due to their very high strength-to-weight and stiffness-to-weight ratios. These properties are sought after in materials used in aerospace, engineering, marine industries, and automobile industries [2].
In this paper, the synthesis and properties of polymer matrices, particularly thermosetting and thermoplastic resins, are reviewed in detail. In addition, the applications of polymer matrices for CF-reinforced polymer composites are discussed.
Cyanate ester (CE) resins are a class of high-performance thermosetting resins consisting of esters that contain at least two cyanate functional groups [1,2]. Most commercial monomers can be represented by the structural model illustrated in Fig. 1.
CE resins can be cured by heating at elevated temperatures or they can be cured at lower temperatures in the presence of a suitable catalyst. The most common catalysts are transition metal complexes such as cobalt, copper, manganese, and zinc complexes. The chemistry of the curing reaction for CE resins is the trimerization of the three CN groups to form a triazine ring. The CE resins have two cyanate groups that facilitate the formation of a three-dimensional polymer network structure [3].
CE resins homopolymerize into a thermosetting material suitable for use in higher-performance composites such as structural composites and those for printed circuit boards and radomes. These resins have good processability, shelf life, and compatibility with a variety of reinforcements. Fully cured bisphenol-E CE has a glass transition temperature (Tg) and a
decomposition temperature (Td) at 5 wt% loss of 274℃ and 438℃, respectively [4,5].
Marieta et al. [2] studied the effect of surface treatment on the interfacial shear strength (IFSS) of bisphenol-A dicyanate (DCBA)/PAN-based CF composites. Fig. 2 shows the apparent IFSS values of the composites with different treatments. The results indicated that the commercial sizing of epoxy was effective in promoting adhesion because of the chemical reactions taking place between the epoxy sizing and DCBA during the curing process.
Epoxy resins are low-molecular-weight pre-polymers containing more than one epoxide group:
Thermosetting epoxy resins are cured using a wide variety of curing agents via curing reactions. Epoxy resins have a wide range of applications including their use in fiber-reinforced materials, general-purpose adhesives, high-performance coatings, and encapsulating materials.
2.2.1. Bisphenol-A epoxy resins
Diglycidyl ether of bisphenol-A (DGEBA) is produced by reacting epichlorohydrin with bisphenol-A in the presence of a basic catalyst. Fig. 3 shows the chemical structure of DGEBA.
The properties of the DGEBA resins depend on the value of n, which is the number of repeating units and is commonly known as the degree of polymerization. Low-molecularweight molecules tend to be liquids and higher-molecularweight molecules tend to be more viscous liquids or solids. DGEBA has been used commercially as a raw material and is the primary chemical building block for a broad spectrum of materials [6,7].
Park et al. [8] demonstrated the effect of fiber-polymer interactions on the fracture toughness behavior of CF-reinforced DGEBA epoxy matrix composites. Fig. 4 shows the evolution of the critical stress intensity factor (KIC) of DGEBA/CF composites as a function of electric current density. The KIC of the composites continually increases with increased current densities of the treatments up to 0.4 A/m2. This is due to the increased interfacial adhesion between the fibers and matrix.
2.2.2. Cycloaliphatic epoxy resins
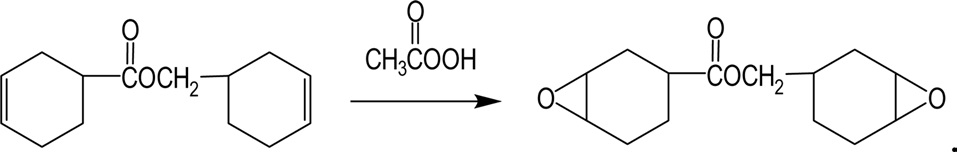
The cycloaliphatic epoxy resin, 3',4'-epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate is synthesized by reacting 3'-cyclohexenylmethyl 3-cyclohexenecarboxylate with peracetic acid, as shown in Fig. 5.
The cycloaliphatic epoxy resin has an aliphatic backbone and a fully saturated molecular structure, which are contributing factors relating to its excellent UV stability, good weatherability, and excellent electrical properties. This epoxy resin, which has a high epoxide content, also exhibits a high crosslink density, Tg, and heat distortion temperature. These properties are crucial for resins used to fabricate structural components requiring high-temperature service. Since this is a short chain epoxy resin, it has a low viscosity, which allows for rapid fiber wet-out in commonly used processes
that include filament winding, pultrusion, and resin transfer molding [9].
2.2.3. Trifunctional epoxy resins
A trifunctional epoxy resin, trimethylol propane-N-triglycidyl ether, is prepared by the reaction of trimethylol propane and epichlorohydrin, as shown in Fig. 6.
The epoxy resin is a low-viscosity, non-crystalline, plastic material, and it can be cured at low temperatures [10,11].
2.2.4. Tetrafunctional epoxy resins
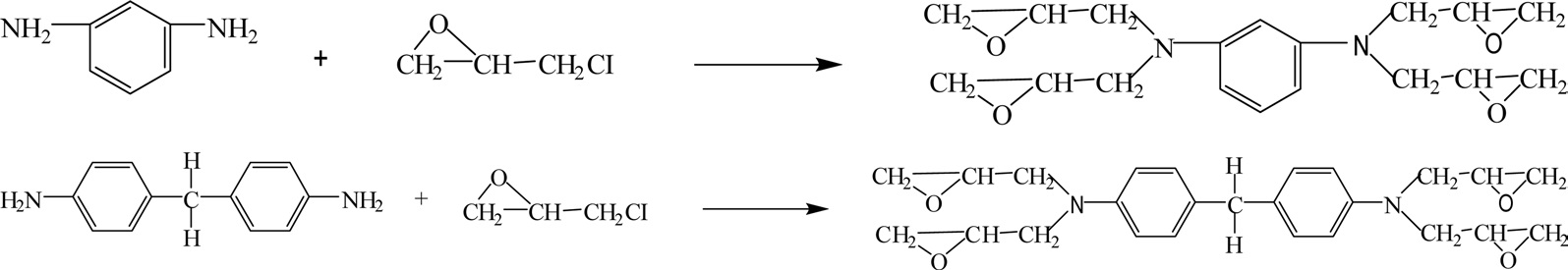
Tetrafunctional epoxy resins are synthesized by reacting 1,3-diaminobenzene or 4,4'-aminodiphenyl methane with epichlorohydrin, as shown in Fig. 7.
Tetrafunctional epoxy resins with high epoxy functionality have high cross-linking densities, and they are used in applications where high-temperature resistance is required. The cured epoxy resins show excellent chemical resistance, high modulus, good UV-blocking effect, and good thermal stability [12,13].
Chen et al. [14] studied the DGEBA/CF and 4,4'-tetradiglycidy diaminodiphenol methane (TGDDM)/CF filament wound composites. The results indicated that the two kinds of resins (DGEBA and TGDDM) with the hardener 4,4'-diaminodiphenyl methane (DDM) or DDM/diethylto1uene diamine (DETDA) had higher tensile properties than the resins cured with the methyltetrahydrophthalic anhydride (MeTHPA) hardener. The TGDDM resin casts showed tensile strengths close to those of the DGEBA resin casts, but the former had longer elongations.
2.2.5. Novolac resins
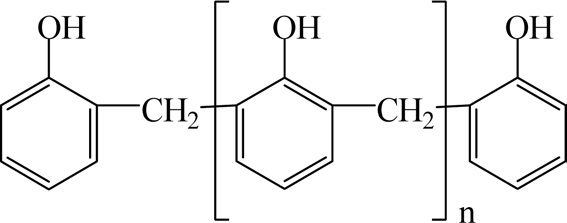
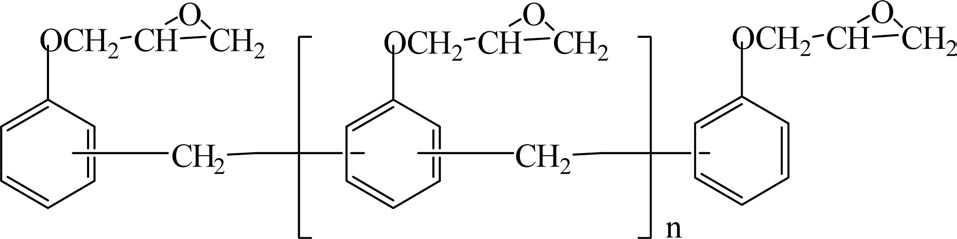
Novolac resins are synthesized by the reaction of a molar excess of phenol with formaldehyde under an acidic catalyst such as oxalic acid, hydrochloric acid, or sulfonic acid. Fig. 8 shows the chemical structure of novolac resins.
Novolac resins are solid at room temperature and will soften and flow between 65℃ and 105℃. The number average molecular weight (Mn) of a standard phenol novolac resin is between 250 and 900. Novolac resins are soluble in many polar organic solvents such as alcohols and acetone, but they are insoluble in water [15].
2.2.6. Novolac epoxy resins
Novolac epoxy resins are glycidyl ethers of phenolic novolac resins that have been synthesized by reacting phenolic novolac resin with epichlorohydrin. Fig. 9 shows the chemical structure of novolac epoxy resins. Because an additional curing agent is required to complete the curing of the resin, the industry commonly refers to novolac resins as "two-step" products.
The multiple epoxide groups in novolac epoxy resins contribute to their high cross-linking densities, which results in their excellent thermal, chemical, and solvent-resistance properties. Novolac epoxy resins are widely used to formulate the molding compounds for microelectronics packaging because of their superior performance at elevated temperature, excellent moldability characteristics and mechanical properties, superior electrical properties, and heat and humidity resistance [16,17].
2.2.7. Epoxy curing agents
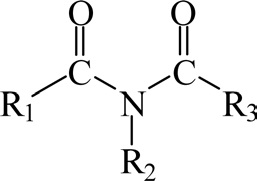
A wide variety of curing agents are used for curing epoxy resins. The cure kinetics and Tg of cured epoxy resins are dependent on the molecular structure of the curing agents. The stoichiometry of the epoxy/curing agent system will be reflected in the properties of the cured epoxy resins. An agent that is not consumed in the reaction is known as a catalytic curing agent. A reactive curing agent is consumed in the reaction and is generally used in much greater amounts than catalytic curing agents. In this case, the curing agents react with the resin molecules, and they are coupled directly into the cured system as structural members of the polymer.
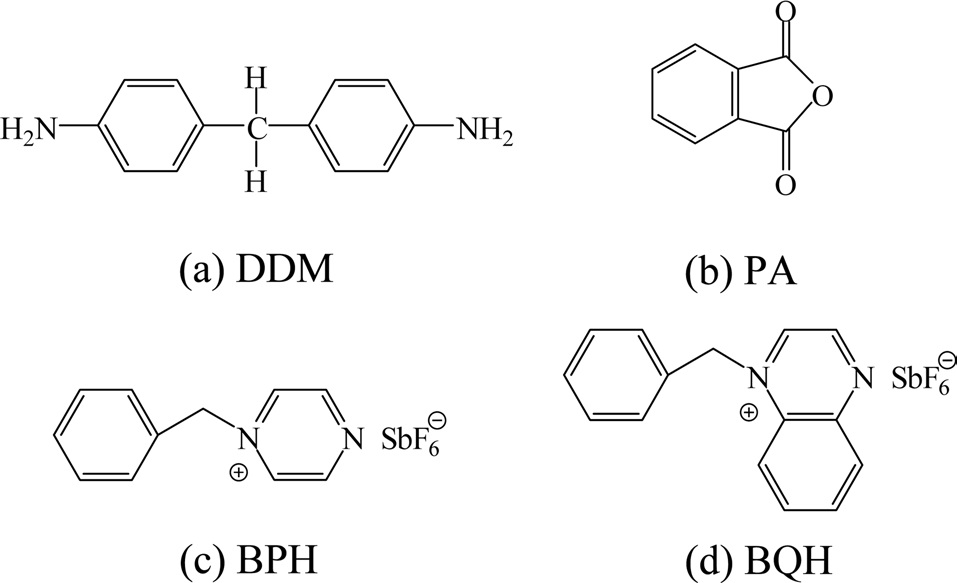
Epoxy curing agents can be divided into amine-type curing agents (such as triethylenetetramine and DDM), alkali curing agents (such as imidazole type and tertiary amines), anhydrides (such as phthalic anhydride [PA] and nadic methyl anhydride), and catalytic curing agents (such as
2.2.8. Curing process
The epoxy curing process is a chemical reaction in which the epoxide groups in the epoxy resins react with a curing agent to form a highly cross-linked, three-dimensional network [22-24].
1) Room-temperature curing
Epoxy resin curing that takes place at room temperature uses curing agents (such as aliphatic polyamine). Room-temperature curing provides a lower Tg, higher flexibility, greater impact resistance, and greater electrical and thermal shock resistance.
2) Heat curing
Generally, epoxy resins are cured with curing agents that react at elevated temperatures. These cured epoxy resins have a higher Tg, greater tensile strength, higher heat resistance, and greater chemical resistance.
3) Photocuring
Epoxy resins can be cured using a photoinitiator. The curing reaction takes place in the presence of infrared or ultraviolet light, or by placing the reactants in the path of an electron beam.
Phenolic resins are normally prepared from phenol and formaldehyde. Phenolic resins can be divided into either resol or novolak resins according to the preparation methods used [25-28].
2.3.1. Resol resins
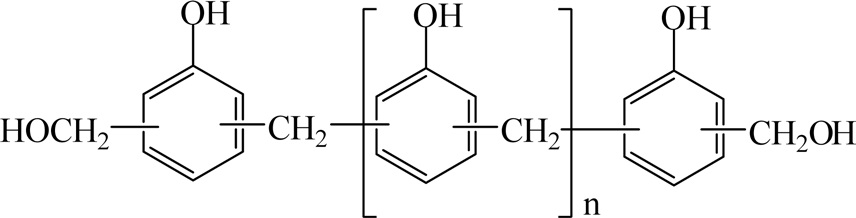
Resol resins were synthesized by reacting a molar excess of formaldehyde with phenol in the presence of a catalytic base. Fig. 11 shows the chemical structure of resol resins.
When an excess of formaldehyde is used in the synthesis of resol resins, a sufficient number of methylol and dibenzyl ether groups remain reactive, i.e., available for completing the polymerization and curing the resin without the need for incorporating an additional cure agent such as hexamethylenetetramine. For this reason, the industry commonly refers to resol resins as "single-stage" products. The typical Mn of a straight phenol resol resin is between 200 and 450.
2.3.2. Novolak resins
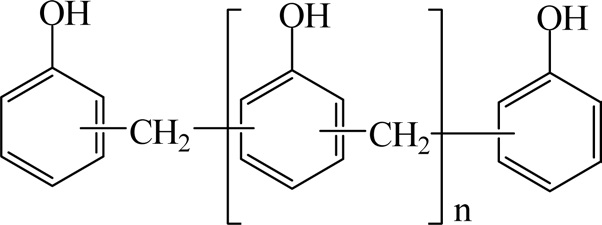
Novolak resins were synthesized by reacting a molar excess of phenol with formaldehyde in the presence of a catalytic acid. Fig. 12 shows the chemical structure of novolak resins.
The reaction creates a methylene bridge at either the ortho position or the para position of the phenolic aromatic rings. These phenolic resins are unable to react further without the addition of a curing agent. Because an additional agent is required to complete the curing of the resin, the industry commonly refers to novolac resins as "two-stage" products. The most common curing agent for novolak resins is hexamethylenetetramine.
Park and Jang [26] demonstrated the interfacial characteristics and fracture toughness of electrolytically Ni-plated CFreinforced phenolic resin composites. Fig. 13 shows the results of the KIC tests of the composites as a function of current density. The KIC increased with an increasing current density up to 10 A/m2. This high fracture toughness can be attributed to the presence of nickel functional groups and a higher number of oxide functional groups present on the CF.
Polyester resins are unsaturated resins formed from the reaction of dibasic organic acids with polyhydric alcohols, as shown in Fig. 14.
Polyester resins have relatively good resistance to UV radiation. They are long lasting and highly water resistant. These resins comprise the most commonly used polymer matrix in the marine and composite industries. The resins are used in sheet molding compounds, bulk molding compounds, and in the toner
used in laser printers. They are commonly used in auto repair, casting, adhesives, and wood filling. These resins can be used with any type of fiberglass CF or Kevlar [27-29].
Polyester resins are classified as thermosetting resins, and they can be cured exothermically to a solid with the addition of the curing agent. Generally, organic peroxides such as benzoyl peroxide and methyl ethyl ketone peroxide (MEKP) are used as the curing agents.
Some commercially important linear polyesters include polyglycolic acid, polylactic acid, polycaprolactone, polyethylene terephthalate, and polybutylene terephthalate.
Vilcakova et al. [32] demonstrated the electrical conductivity of polyester resin/CF composites in the percolation threshold region. A steep conductivity increase at a relatively low CF content (0.7 vol%) was observed, which was thought to be caused by a special mechanism involved in the formation of the intrinsic fibrous structure of the composites.
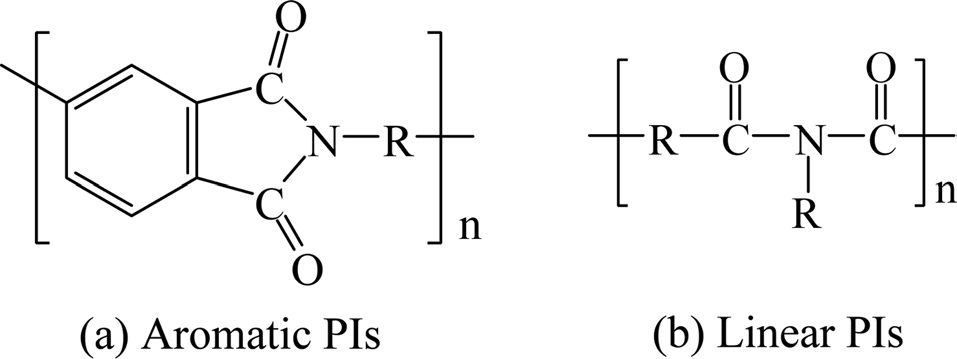
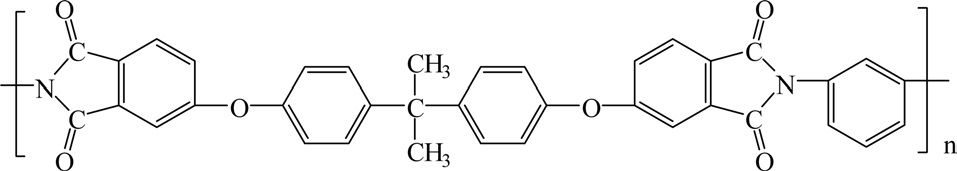
Polyimide (PI) is a polymer constructed from imide monomers. Fig. 15 shows the structure of PI [33-35].
PIs can be divided to aliphatic PIs where the atoms of the imide group are part of a linear chain and aromatic PIs where the imide group is part of a cyclic unit in the polymer chain, as shown in Fig. 16.
PIs possess a greater resistance to heat than any other unfilled organic material. Unlike most plastics, PIs are available in laminates and shapes, molded parts, and stock shapes. PI parts are fabricated by techniques ranging from powder-metallurgy methods to conventional injection, transfer, compression molding, and extrusion.
PIs have low dielectric constants, and they can be polishable to an angstrom-level surface finish. In addition, they have excellent dimensional stability and flexibility, and they exhibit low water absorption. They have high temperature
stability, excellent machinability, exceptional mechanical strength, and low coefficient of thermal expansion, and they exhibit low outgassing. In addition, they are non-contaminating. They are used in place of metals and glass in many highperformance applications in electronics, automotive design, and the aerospace industry.
PIs are solid, heat-resistant, incombustible substances of predominantly amorphous structure, with Mn of 50 000-150 000 and a density of 1.35-1.48 g/cm3 at 20℃. Most PIs do not dissolve in organic solvents and are inert to the action of oils. Further, most remain virtually unchanged under the action of dilute acids. They are hydrolyzed by alkaline substances and superheated steam. PIs are resistant to ozone, γ-rays, fast electrons and neutrons, and are highly resistant to heat.
Li and Cheng [36] studied the friction and wear properties of surface-treated CF-reinforced PI composites under oil-lubricated conditions. As shown in Fig. 17, the friction coefficients of air-oxidized and rare earth solution treated CF-reinforced PI composites are lower than those of untreated CF-reinforced PI composites. This would indicate that surface modification can improve the interfacial adhesion and compatibility between the CF and the PI matrix.
Vinyl ester (VE) is a resin produced by the esterification of an epoxy resin with an unsaturated monocarboxylic acid. These resins combine the advantages of both epoxy and polyester resins. The resins possess the higher flexibility that is observed in epoxy resins with an ease of processing that is like that involved in the synthesis of polyesters [37].
VE resins are stronger than polyester resins and cheaper to make than epoxy resins. VE resins have lower viscosities than
polyester and epoxy resins. VE resins offer better resistance to moisture absorption than polyester resins. It is also known that VE resins bond extremely well to fiberglass but bond poorly to Kevlar and CF due to the nature of those two more exotic fibers [38].
Bisphenol-A epoxy VE resin, as shown in Fig. 18, is resistant to a wide range of acids, alkalis, bleaches, and organic compounds that are commonly used in many industrial chemical processing applications.
VE resins are exceptionally reactive and cure rapidly. Peroxides, such as MEKP, dicumyl peroxide, di(t-butylperoxy) cyclohexane, and tertiary-butyl peroxybenzoate, were used as initiators for curing VE systems [39]. Cured VE is more flexible and has greater fracture toughness than polyester. VE can also withstand temperatures of up to <200℃ without distorting.
Yamada et al. [40] demonstrated the plasma-graft polymerization of a monomer with double bonds onto the surface of CF and evaluated its adhesion to a VE resin. Fig. 19 shows the pullout force of the yarns, which were grafted by poly(adipic acid divinyl ester) chains and were then embedded in the cured VE resin/benzoyl peroxide/
3.1. Acrylonitrile butadiene styrene resins
Acrylonitrile butadiene styrene (ABS) is a terpolymer of acrylonitrile, butadiene, and styrene. Fig. 20 shows the chemical structure of ABS. Many industrial methods such as bulk, bulksuspension,
emulsion, and emulsion graft-doped legitimate are used to prepare ABS resins.
Common ABS resin compositions are about half styrene with the balance divided between butadiene and acrylonitrile. The higher the percentage of acrylonitrile in the ABS resin, the better the heat-resistance, rigidity and solvent-resistance will be. However, the opposite is true for the flow character. The heat-resistance and solvent-resistance for these resins are better than they are for high impact polystyrene (HIPS). The impact strength and the tensile strength as well as the surface hardness of ABS are better than those observed in HIPS. ABS resin is a tough, light, economical, heat- and stain-resistant, excellent engineering plastic [41,42].
Li and Cai [42] studied the CF surface treatment and the addition of PA6 on the tensile properties of ABS composites. Fig. 21 shows the effects of the short CF (SCF) content on the tensile properties of ABS/SCF composites. Increasing the SCF content increases the tensile strength and modulus of the composites, which is in agreement with the well-known equation of Kelly and Tyson for mixing short fiber-reinforced composite materials.
Polyamide (PA) is a polymer containing monomers of amides joined by peptide bonds. PAs are resins that include a CO-NH functional group in their polymer backbone. They may be produced
by the interaction of an amine (NH2) group and a carboxyl (COOH) group, or they may be formed by the polymerization of amino acids or amino-acid derivatives. They can occur both naturally and artificially. PAs are commonly used in textiles, automotive, carpet, and sportswear because of their extreme durability and strength [43,44].
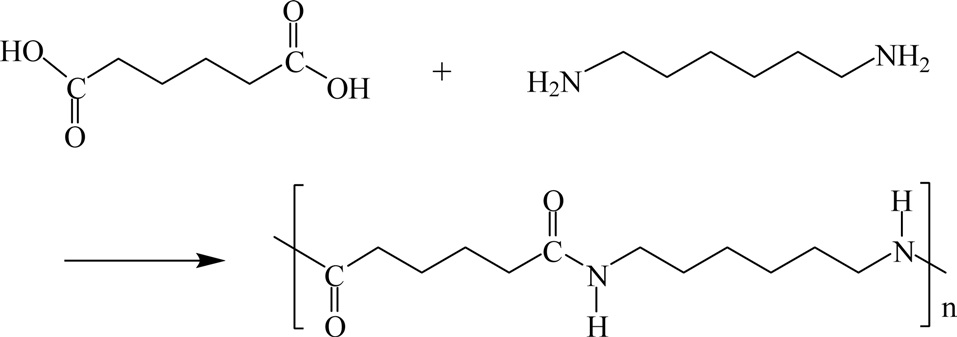
Poly(hexamethylenediamine adipamide) (Nylon 66, PA66) is one of the most widely used semi-crystalline engineering thermoplastic resins. Nylon 66 is synthesized by reacting adipic acid with hexamethylene diamine, as shown in Fig. 22.
Nylon-66 is a semicrystalline polyamide commonly used in fiber applications such as carpeting, clothing, and tire cord. It is also used as an engineering material in bearings and gears due to its good abrasion resistance and self-lubricating properties [45]. Nylon 66 has good sliding properties, is abrasion resistant, has high tensile strength, a higher melting point, and is electrically insulating. Because of these properties it is widely used in gear wheels, friction strips, piston guides, impact plates, cam discs, and much more [46].
Botelho et al. [44] studied the mechanical behavior of CFreinforced PA composites. Their results indicate that an increase in the fiber volume fraction improved the mechanical properties of the composites.
Polycarbonate (PC) is produced by the reaction of bisphenol A and phosgene COCl2. The overall reaction is shown in Fig. 23.
PC has the advantage of having a uniquely high impact strength and exceptional clarity, which makes it a valuable material for use in bulletproof windows, break resistant lenses, compact discs, etc. [47,48]. PCs are used mainly as molding compounds. The commercially important PCs in use include bisphenol A and diphenyl carbonate. These polymers are clear plastics with a slight yellow discoloration. They have excellent electrical properties and high impact strength [49].
Choi et al. [50] demonstrated the production and characterization of PC composite sheets reinforced with vapor grown CF (VGCF). Fig. 24 shows the Young's modulus of PC/VGCF cast and rolling composites reinforced with various amounts of VGCF. The Young's modulus of both the composites increased with an increase in the VGCF content.
3.4. Polyetheretherketone resins
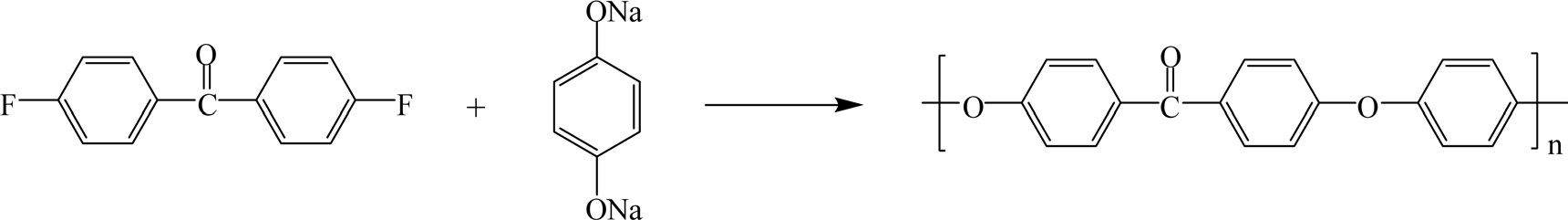
Polyetheretherketone (PEEK) resins are synthesized by stepgrowth polymerization through the dialkylation of bisphenolate salts. Typically, the reaction used is that of 4,4'-difluorobenzophenone combined with the disodium salt of hydroquinone, which is generated in situ by deprotonation by sodium carbonate, as shown in Fig. 25 [51].
PEEK is a semicrystalline thermoplastic with excellent mechanical and chemical resistance properties that are retained even under high temperatures. The Young's modulus is 3.6 GPa and it has a tensile strength of 90 to 100 MPa. PEEK has a Tg occurring at around 143℃ and melts at roughly 343℃. PEEK carries a V-0 flammability rating and exhibits very low smoke and toxic gas emission when exposed to flame [52].
PEEK is generally used as an engineering material as it exhibits high strength and low wear, good resistance to corrosion, and the almost unique ability among polymers to operate effectively in an ultra high vacuum environment [53,54].
Gebhard et al. [55] studied the wear of aqueous lubricated SCF reinforced PEEK composites. Fig. 26 shows the fiber corrosion depths of the composites under different experimental and environmental conditions. The fiber corrosion depth of TGC (after a sliding experiment with simultaneous galvanic corrosion) showed the highest value seen in this study. This means that for TGC there is not only an increase in the wear rate due to the occurrence of fiber corrosion, but also a complimentary effect of tribological stress enhanced CF corrosion.
Polyetherimide (PEI) is an amorphous, engineering thermoplastic resin. Fig. 27 shows the chemical structure of PEI. PEI has high heat resistance, excellent electrical properties, high strength and modulus, and excellent processability. The Tg and amorphous density of PEI at 25℃ are 216℃ and 1.27 g/cc, respectively. Unmodified PEI resin is transparent, and it has inherent flame resistance properties and exhibits low-smoke evolution [56].
PEI can be processed on conventional thermoplastic molding equipment. PEI resin is available for general-purpose injection molding, blow molding, foam molding, and extrusion. PEI is extruded to produce profiles, coated wire, sheet, and film. The addition of glass fiber reinforcement to the PEI provides it with both greater tensile strength and greater rigidity while at the same time improving its dimensional stability [57].
PEI resists a broad range of chemicals under varied conditions of stress and temperatures. PEI is compatible with aliphatic hydrocarbons and alcohols, such as gasoline and gasohol, mineral-salt solutions, dilute bases, and fully halogenated hydrocarbons [58].
Xian and Zhang [59] demonstrated the influence of SCF reinforcement on the sliding wear of PEI composites. Fig. 28 shows the evolution of the specific wear rates with the SCF fraction.
They found that the addition of SCF could reduce the specific wear rate of PEI by around 60 times at room temperature and 80 times at 150℃, which is due to the reinforcing, lubricating, and thermal conducting functions of SCF.
Polyethersulfone (PES) is an excellent heat resistant, transparent, non-crystalline engineering plastic containing ether groups and sulfone groups in its backbone chains. Fig. 29 shows the chemical structure of PES.
PES resins have high temperature performance and a high Tg, good dimensional stability, outstanding rigidity even at high temperature, excellent insulation properties, are biocompatible, and are inherently flame retardant [60].
PES can be molded on conventional plastics processing equipment such as injection molding, extrusion, compression molding, solution casting, and sintering. Because of its amorphous nature, PES has excellent dimensional stability and it can be easily processed with highly polar solvents, so it is suitable for applications requiring close tolerances and little dimensional change over a wide temperature range. PES composites offer distinct advantages over thermosets: shorter processing time, better toughness, reduced storage and scrap, and ability to be repaired [56].
Wu and Schultz [56] studied the processing and properties of solution impregnated CF-reinforced PES composites. The results indicated that a higher molding temperature and longer molding time increased flexural strength from 44 MPa to 71 MPa.
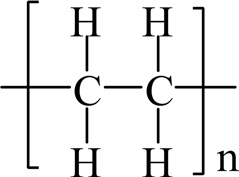
Polyethylene (PE) is a thermoplastic polymer consisting of long chains of the monomer ethylene, as shown in Fig. 30. PE is
a light, versatile synthetic resin made from the polymerization of ethylene. PE can be produced by radical polymerization, anionic addition polymerization, cationic addition polymerization, or ion coordination polymerization. Its simple basic structure can be linear or may be branched to a greater or lesser degree.
PE is a member of the important family of polyolefin resins. It is the most widely used plastic in the world. It has been made into products ranging from clear food wrap and shopping bags to detergent bottles and automobile fuel tanks. It can also be slit or spun into synthetic fibers or modified to take on the elastic properties of rubber [61].
PE is available in a range of flexibilities and physical properties depending on the production process. PE can be formed by a wide variety of thermoplastic processing methods and is particularly useful where moisture resistance and low cost are required.
The mechanical properties of PE depend significantly on variables such as the extent and type of branching, the crystal structure, and the molecular weight. PE is classified into low density PE (LDPE), linear low density PE (LLDPE), high density PE (HDPE), etc. LDPE, LLDPE, and HDPE typically have density values ranging from 0.91 to 0.925 g/cm3, 0.918 to 0.94 g/cm3, and 0.935 to 0.96 g/cm3, respectively [62,63].
Zhang et al. [64] demonstrated the selective location and double percolation of CF filled HDPE/isotactic polypropylene (iPP) blends. Fig. 31 shows the dependence of the electrical conductivity on VGCF content for HDPE, iPP, and HDPE/iPP blends. The HDPE samples exhibit a percolation threshold at 2.5 phr (parts per hundred parts resin) VGCF content, whereas for the iPP samples, the percolation threshold is 1.75 phr VGCF content. This is due to the different filler dispersion states of the two polymers.
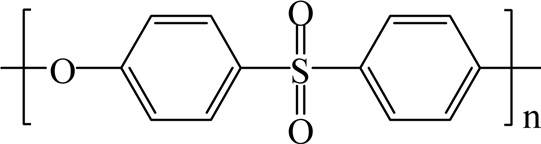
3.8. Polyphenylene sulfide resins
Polyphenylene sulfide (PPS) is formed by the reaction of sodium sulfide with p-dichlorobenzene as shown in Fig. 32. PPS is an organic polymer having a symmetrical rigid backbone chain consisting of recurring p-substituted benzene rings and sulfur atoms. PPS is a semicrystalline, high-performance engineering thermoplastic.
PPS exhibits outstanding chemical resistance, thermal stability, dimensional stability, and fire resistance. PPS's extreme inertness toward organic solvents, and inorganic salts and bases accounts for its outstanding performance as a corrosion-resistant coating suitable for contact with foods [65].
PPS can be molded, extruded, or machined to high tolerances. In its pure solid form, it may be opaque white to light tan in color. PPS resins are available as powders for slurry coating and electrostatic spraying. Because of its low viscosity, PPS can be molded with a high loading of fillers and reinforcements. This is needed to compensate for its inherent brittleness.
Xu et al. [66] studied the tribological behavior of PPS/CF composite coating under dry sliding and water lubrication. Fig. 33 shows the variations in the friction coefficient of the composite coating with the content of CF at a sliding speed of 0.43 m/s under 200 N. It showed that the friction coefficient of the composite coating appeared to decrease slightly with increasing CF content both under dry- and water-lubricated conditions. At the same time, the friction coefficient under the water-lubricated condition was much lower than that under dry sliding.
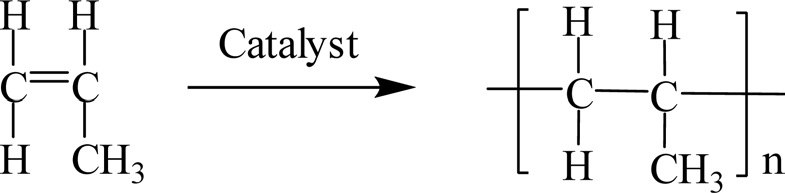
Polypropylene (PP) is a thermoplastic polymer with a linear structure made from the monomer propylene by Ziegler-Natta
polymerization and by metallocene catalysis polymerization as shown in Fig. 34.
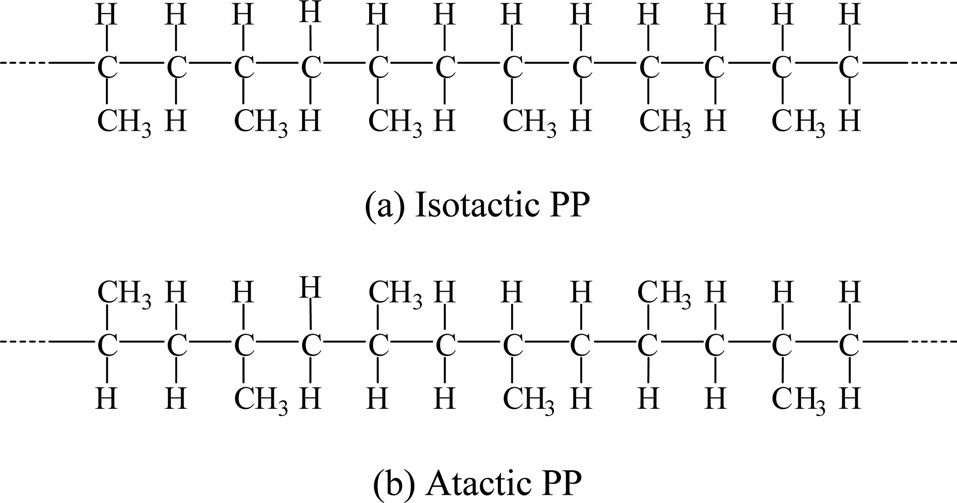
Short segments of PP show examples of isotactic and syndiotactic tacticity as shown in Fig. 35.
PP resins are cheap and have low densities (0.90-0.92 g/cm3) and high tensile and compressive strength. They have a melting point of 160℃, excellent dielectric properties, and are nontoxic. They are used in a wide variety of applications including packaging and labeling, textiles, stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes. In addition, these polymers made from the monomer propylene are unusually resistant to many chemical solvents, bases, and acids [67-69].
In general, homopolymers can be used for housing, housewares, packaging, cassette holders and fibers, monofilaments, and film tapes; copolymers are preferred for all applications exposed to cold. Copolymers are widely used for pipes, containers, boat hulls, seat shells, and automotive parts.
In order to improve some of the physical properties of PP, PP formulas may include additives, such as pigments, carbon black, rubbers, antioxidants, and UV stabilizers. PP is available as molding powder, extruded sheet, cast film, textile staple, and continuous filament yarn [70-72].
Li [73] demonstrated the interfacial compatibility of PP composites filled with surface treated CF. Fig. 36 shows the threepoint bending value of PP/CF composites as a function of treatment time. The bending strength and modulus increase with an increasing treatment time, which indicates that the interfacial adhesion between the CF and the PP matrix increases with treatment time.
In this paper, we reviewed the synthesis and properties of thermosetting and thermoplastic resins. The role of the polymer matrix in CF-reinforced composites is to provide bulk to the composite laminate and transfer load between fibers. Selection of a suitable polymer matrix plays an important role in the specific applications of the composites. CF-reinforced polymer composites are of great interest as multifunctional high-performance materials for use in structural and thermal protection applications.

![Apparent IFSS data of DCBA/carbon fiber composites with different treatments by pull out measurements [2]. IFSS: interfacial shear strength, DCBA: bisphenol-A dicyanate.](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f002.jpg)

![Effect of current density on KIC of diglycidyl ether of bisphenol- A/carbon fiber composites [8].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f004.jpg)
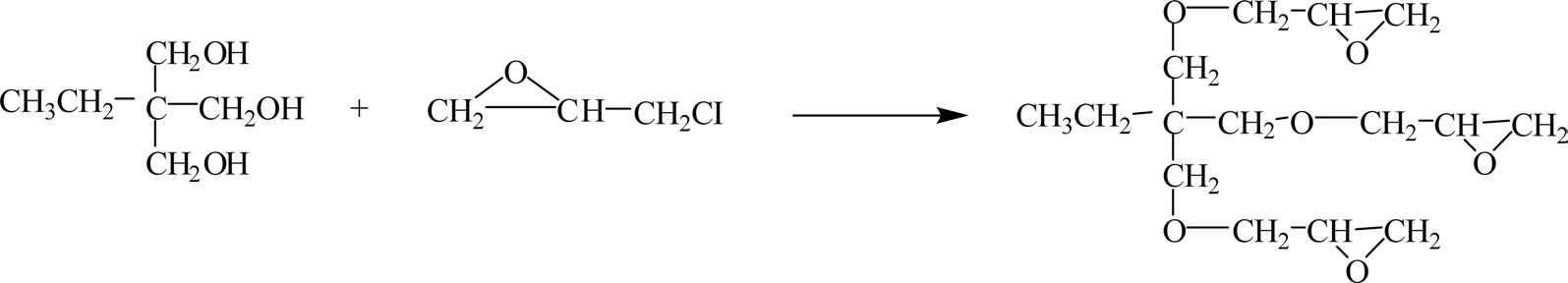
![Evolution of KIC of phenolic resin/carbon fiber composites with current density [25].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f013.jpg)
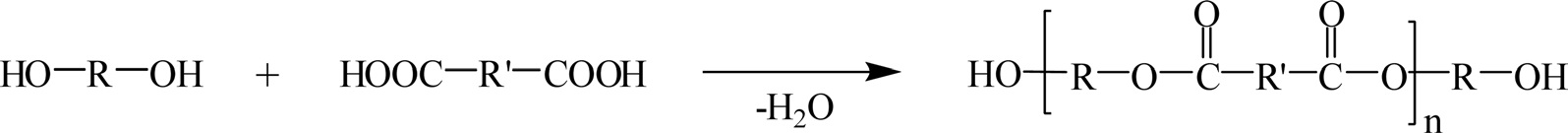
![Effect of surface treatment on friction of polyimide/carbon fiber composites [36].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f017.jpg)

![Pull-out force of the grafted carbon yarn as a function of the degree of grafting in adipic acid divinyl ester [40].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f019.jpg)
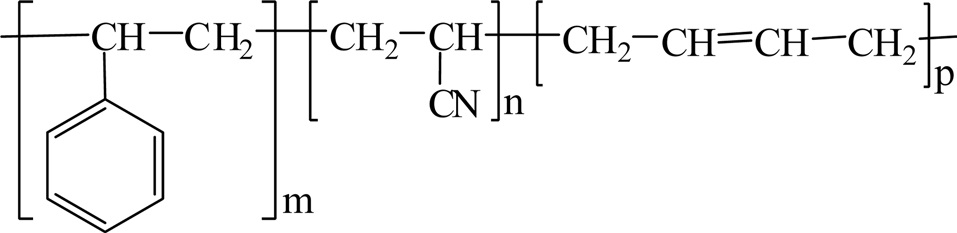
![Tensile properties of acrylonitrile butadiene styrene/short carbon fiber (SCF) composites [42].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f021.jpg)
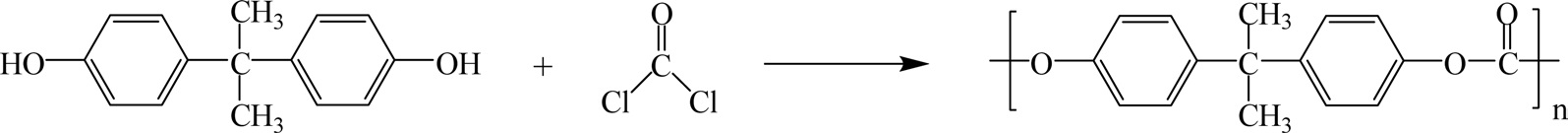
![Young’s modulus of polycarbonate/vapor grown carbon fiber (VGCF) cast and rolling composites reinforced with various amount of VGCF [50].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f024.jpg)
![Fiber corrosion depths of polyetheretherketone vs. stainless steel pairing under different experimental and environmental conditions [55]. T: pure sliding, GC: galvanic corrosion, T+GC: mathematical addition, TGC: sliding experiment with simultaneous galvanic corrosion.](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f026.jpg)
![The specific wear rate of neat polyetherimide and its short carbon fiber (SCF) composites at room temperature and 150℃, measured with a pin-on-disc testing rig, 1 m/s, and 2 MPa [59].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f028.jpg)
![Dependence of electrical conductivity on vapor grown carbon fiber (VGCF) content for VGCF filled high density polyethylene (HDPE), isotactic polypropylene (iPP), and HDPE/iPP (50/50) blends molded at 190℃ for 15 min [64].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f031.jpg)
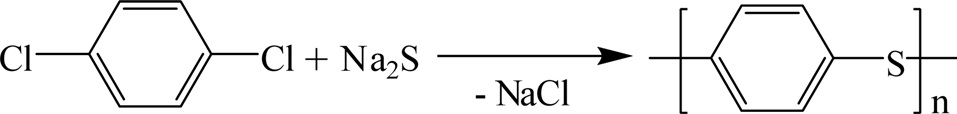
![Variations in friction coefficient of polyphenylene sulfide/carbon fiber (CF) composite coating as a function of CF content [66].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f033.jpg)
![Bending strength and modulus of polypropylen/carbon fiber composites [73].](http://oak.go.kr/repository/journal/11910/HGTSB6_2013_v14n2_76_f036.jpg)