The direct methanol fuel cell (DMFC) is an extremely promising power source for portable applications due to its simple handling and processing of fuel. DMFCs have several advantages (e.g., high energy-conversion efficiency, simple design, and environmental friendliness) compared with hydrogen, for applications involving transportation and portable electronic systems. Such fuel cells can provide a clean power source with high theoretical energy density and low cost, without requiring a separate hydrogen generation system [1]. Despite many efforts devoted to development of the DMFC, there still remain difficulties to be overcome in terms of efficiency and power density. Commercialization of these fuel cells has been facing serious difficulty due to kinetic constraints on the methanol oxidation reaction. To this end, Pt-based electro-catalysts for the methanol electro-oxidation reaction in acid solution has been widely studied in recent years, but high cost and limited quantities have prevented Pt from being used at commercial levels [2]. In addition, the Pt electrocatalyst is poisoned by intermediate products of methanol oxidation (e.g., COads). Since the mid-1970s, modification of the catalyst surface has been made by the addition of a second, less expensive metal to the platinum to selectively direct methanol electro-oxidation to the platinum particles [3]. The electro-catalytic activity of Pt-based catalysts can be substantially enhanced by incorporation of other metals or oxides to form binary-alloy catalysts (i.e., PtRu, PtSn, PtFe, and PtCo) [4-6]. Conductive polymers, with highly accessible surface areas and high stability, have also been used to improve the activity of the graphene support layer and to enhance dispersal of the metal [7]. With this in mind, we developed a facile and efficient way to deposit Pt nano-particles using polypyrrole, on reduced graphene oxide (RGO) functionalized with conducting polymer. All the results demonstrated that, with high electronic conductivity and easier charge transfer, this Pt/PPy-RGO combination exhibited the higher activity and stability than widely used commercial catalysts.
Graphite oxide was synthesized from natural graphite (SP-1, Bay carbon) by a modification of Hummer’s method [8]. The graphite powder was added to a mixture of sulfuric acid, sodium nitrate, potassium permanganate for the acid treatment. The oxidized and treated solution was filtered and washed with HCl (10%); then subjected to centrifugation (3600 rpm, 5 min) to remove residual graphite. The product of synthesis of PPy-RGO composites from 35 mg of graphene and 20 mg of pyrrole, was homogenously dispersed in ethanol-water solution (1:1), then excess (NH4)2S2O8 was slowly added to the suspension, which was stirred continuously at 0-5℃ for 8 h. Finally, the resulting PPy-graphene powder was filtered and rinsed, first with water; then with ethanol, until the filtrate was colorless. The black powder so obtained was freeze-dried overnight. The product of synthesis of the Pt/PPy-RGO composites, using Pt/PPy-RGO at 20 wt% Pt loading, was then used as seeds. The PPy-functionalized RGO (0.1 g) was mixed ultrasonically with H2PtCl6?6H2O in ethylene glycol, after which the pH was adjusted to 10-11 by adding NaOH (1.0 M). The solution was subjected to ultrasonication for 10 min, and N2 bubbling for 20 min; then stirred for 4 h at 120℃. After reaction, the resulting solid, black product was isolated by centrifugation, washed with distilled water and ethanol to remove any remaining ions in the final products, and then freeze-dried overnight. This final product is called PPy-RGO supported, Pt nano-particles.
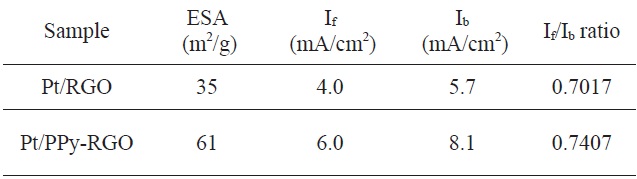
The morphology of the Pt/PPy-RGO product and the deposition of the Pt nano-particles were characterized by transmission electron microscopy (TEM). In Fig. 1a, sheet-like graphene with crimple can be observed. In Fig. 1b, these PPy-assisted, graphene nano-sheets were exfoliated from graphite, and exhibited a smooth, paper-like structure. TEM images of Pt/RGO and Pt/PPy-RGO are shown in Figs. 1c and d, respectively. In Fig. 1c, the Pt/GO combination is shown condensed with other platinum nano-particles, which caused an increase in particle diameters and subsequent decrease in the activity at the Pt nano-particle sites, compared with those of the Pt/PPy-RGO combination.
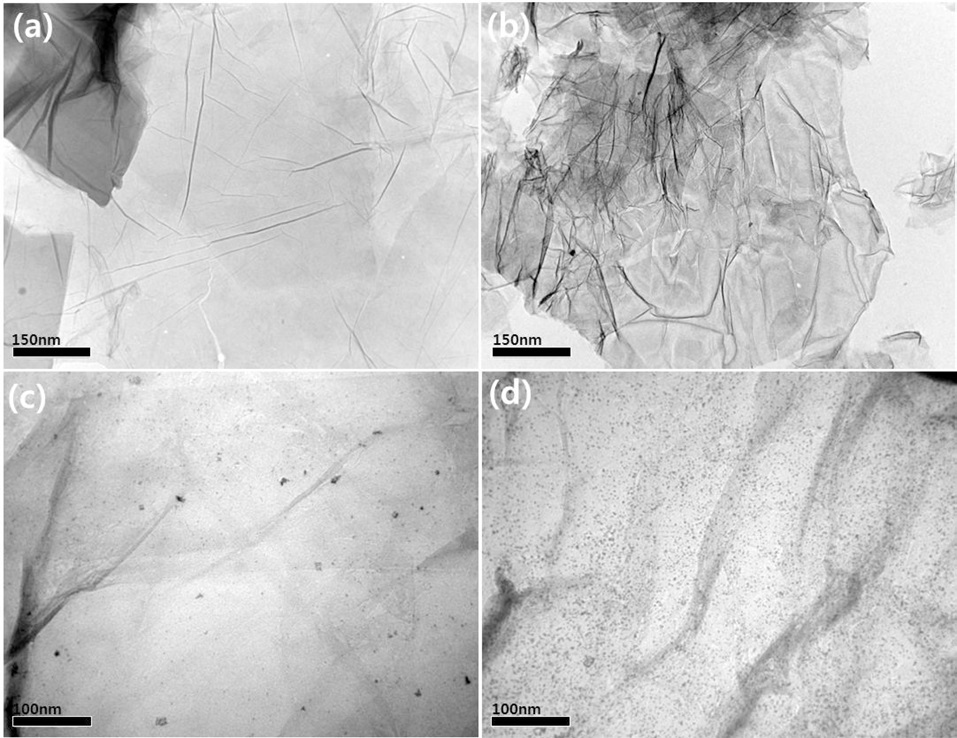
The X-ray diffraction (XRD) patterns of the Pt/RGO (a) and Pt/PPy-RGO (b) in Fig. 2 revealed distinguishing diffraction peaks. The graphite showed (002) a diffraction peak at 2θ = 26.5° [9], but for Pt/PPy-RGO, the diffraction peaks of C (002) plane-shifted negatively. These peaks appeared at 24.8° and 24.1°, corresponding to an interlayer space of 0.337 nm, and implied successful preparation of the PPy-RGO. The diffraction peaks at 2θ = 40.2°, 46.4° and 68.2° could be attributed to the (111), (200) and (220) crystalline planes of the face-centered cubic structure, respectively, of the Pt [10]. The Pt (220) peaks can be used to calculate the average Pt particle size according to the Scherrer equation [11]:
Where d is the average particle size (nm), λ is the wavelength of the X-ray used (0.15406 nm), B2θ is the width of the diffraction peak at half height in radians, and θmax is the angle at the position of the peak maximum. The calculated mean sizes of Pt particles were found to be 2.7 and 2.4 nm for the Pt/RGO and Pt/PPy-RGO electro-catalysts, respectively.
Fig. 3 shows the CV of Pt/RGO and Pt/PPy-RGO in 0.5 M H2SO4 solution at 20 mV/S in a potential window of -0.2 to 1.0 V vs. SCE. Well-defined hydrogen desorption and adsorption peaks in the potential range of -0.05 to +0.3 V, and irreversible pre-oxidation and reduction peaks in the potential range of +0.5 to +1.0 V were observed. The electrochemically active surface area (EAS) was determined by assuming that a monolayer of adsorbed hydrogen requires 210 μC/cm2 for its oxidation [12]. The EAS was then calculated using the following equation [13].
Where ‘S’ is the proportionality constant used to relate charge with area and ‘
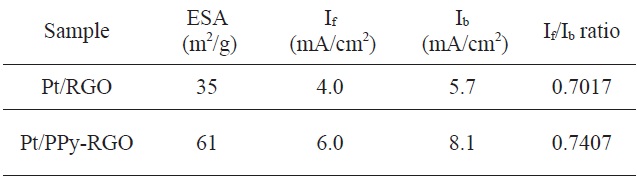
[Table 1.] The cyclic voltammetry results for Pt/RGO synthesized by different mechanisms
The cyclic voltammetry results for Pt/RGO synthesized by different mechanisms
Ib. The higher anodic current of Pt/PPy-RGO indicated that the electric conductivity of graphene was higher than the commercial carbon, a result consistent with previous reports [15]. All of the above data reveal that the Pt/PPy-RGO combination exhibits enhanced catalytic activity for methanol oxidation.
In summary, a convenient and green approach was used to synthesize Pt nano-particles on polypyrrole-assisted graphene for methanol oxidation. XRD and TEM were used to characterize the morphology and structure of the catalysts. The electrocatalytic activities were measured by CV. In comparison with graphene as the support for the catalyst, the polypyrrole-assisted graphene can effectively enhance the electro-catalytic activity of the Pt nanoparticles for the oxidation of methanol. This was due to an increase in the conductivity of the graphene, by the insertion of polypyrrole. All the results indicated that the Pt/PPy-RGO combination is the more promising catalyst for fuel cell applications.
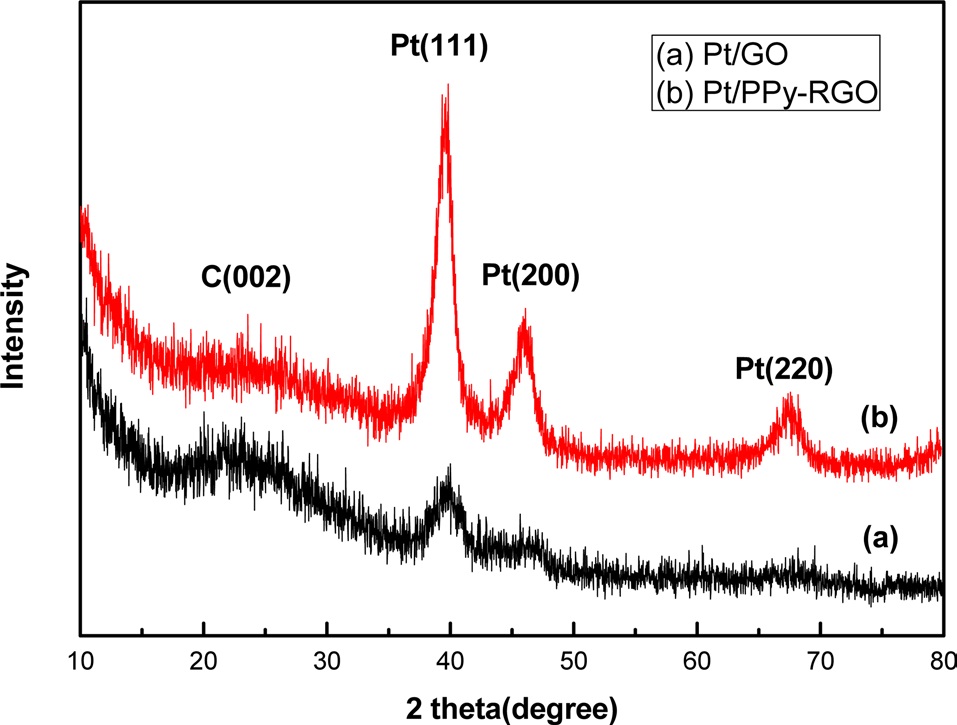
![(a) Cyclic voltammetry (CV) of Pt/RGO and Pt/PPy-RGO in 0.5 M H2SO4 solution at a scan rate of 50 mVs01 [<<verify unit] between -0.2 and 1.0 V vs. SCE, (b) CV of Pt/RGO and Pt/PPy-RGO in 0.5 M H2SO4 and 1 M CH3OH solutions, respectively, at a scan rate of 50 mVs-1 between -0.2 V and 1.0 V vs. RGO: reduced graphene oxide, SCE: saturated calomel electrode.](http://oak.go.kr/repository/journal/11906/HGTSB6_2013_v14n2_117_f003.jpg)