The occasional use of pesticides in large amounts may cause environmental pollution and therefore be a cause of concern. Residual amounts of organochlorine and organophosphate pesticides have been detected in soil, water reservoirs, vegetables, grains and other food products [1]. Organophosphates induce toxicity in mammals through inhibition of acetylcholinesterase (AchE) and subsequent activation of cholinergic receptors [2]. Oxidative stress as a result of organophosphate exposure has been reported [3], and lipid peroxidation has been revealed to be one of the molecular mechanisms in organophosphate-induced toxicity [4,5]. The use of natural remedies has been recently observed to be a good means of curbing oxidative stress by boosting the natural antioxidant defence system [4].
In Africa, medicinal plants are used traditionally to treat various ailments. Among the promising medicinal plants,
Dimethoate emulsifiable concentrate was purchased from Agrivet PTY (LTD) Botswana. Other chemicals were of analytical grade and were bought from Sigma-Aldrich (St. Louis, USA). The kits were purchased from Aggape diagnostics, Kerala-India. The ZM fruits were collected around Gaborone. The plant authentication was performed by Mr. M. Muzila, curator, Hebarium Sector of the University of Botswana. Fruits were washed with distilled water and sun dried. The dried fruits were ground with a laboratory grinder to obtain 500 g of powder. The powder was soaked and extracted in hexane, 50% hexane/chloroform, chloroform, 50% chloroform/methanol, methanol and 70% methanol/water for 24 hr. The extract was made solvent free in rotary vaporizer at reduced pressure. The final extract yield was 25% w/w.
Thirty male Sprague Dawley (SD) albino rats aged 20-21 weeks were kept in galvanized cages under a controlled temperature of 25℃ and a normal photoperiod (12-h dark, 12-h light). The animals were fed commercial food pellets (protein: 18%, fat: 6%, fiber: 6%, carbohydrates: 56%, calcium: 0.6% moisture: 10%) and water ad libitum. All procedures with the animals were performed in accordance with the protocols established by the University of Botswana Animal Care Committee (Approval no: UB/IRB/1084). The thirty adult male rats were divided into six groups of 5 rats each and treated as follows:
Group 1 - normal control (NC) received distilled water,
Group 2 - dimethoate control (DC)received 6 mg/kg.bw.day-1 dimethoate dissolved in distilled water,
Group 3 - experimental group (E1)received dimethoate(6 mg/kg.bw) + ZMFM (100 mg/kg.bw-1),
Group 4 - experimental group (E2) received dimethoate (6 mg/kg.bw) + ZMFM (200 mg/kg.bw-1),
Group 5 - experimental group (E3) received dimethoate (6 mg/kg.bw) + ZMFM (300 mg/kg.bw-1),
Group 6 - experimental control (EO) received ZMFM (300 mg/kg.bw-1) only.
The experiment was run for 90 days, and all extracts were administered orally with the help of a rubber tube and syringe. At the end of the experiment, rats were sacrificed after having been treated with mild ether anaesthesia.
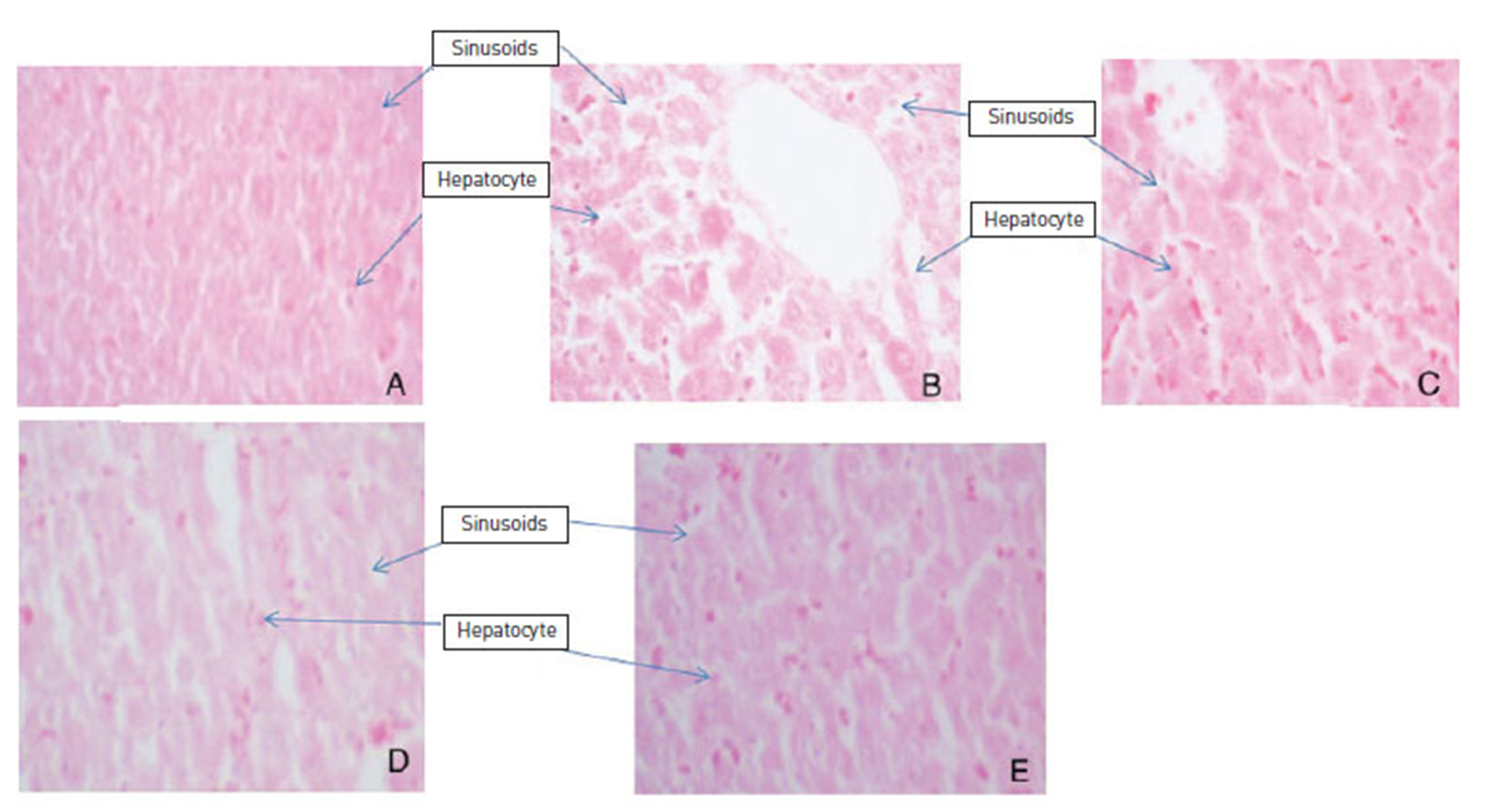
Blood was collected in heparinized tubes and centrifuged at a rate of 6000 rotations per minute, after which plasma samples were drawn and stored at -70℃ for further analysis. The rat liver tissue was removed and immediately fixed in 10% phosphate-buffered formalin for 24 hr. The tissue was dehydrated in an ethanol series (70%, 80%, 100%, and 100%), cleared in xylene and embedded in paraffin. Five-㎛-thick sections were taken by using a rotary microtone, and sections were affixed on clean glass slides by gentle heating. The sections were deparaffinised, stained with haematoxylin and eosin, and mounted with a cover slip by using neutral mounting media (DPX). The slides were observed under a light microscope for any histological changes at x400 [16].
The 2, 2-diphenyl-1-picryl hydrazyl radical (DPPH) semiquantitative assay was performed on thin layer chromatography according to the method described by Yeboah and Majinda [7], and the spectrophotometric measurement of free-radical scavenging activities of the extracts by DPPH was done using the method described by Stoilova et al. [8]. The 2.4.3. 2, 2-Azobis-3-ethyl benzothiazoline-6-sulphonic acid (ABTS) radical scavenging activity. (ABTS) was determined as described by Pellegrini et al. [9], and the total phenol content (TPC) was determined by using the method described by Stoilova et al. [8].
Activities of serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) and alkaline phosphatase (ALP) and measurements of the levels of plasma low-density lipoproteins (LDL), highdensity lipoproteins (HDL), total protein (TP), triglyceride (TG) and cholinesterase activity were done using kits from Aggape Diagnostics, India. Thiobarbaturic acid reactive substances (TBARS) were measured by using the method described by Chaturvedi [10], and reduced glutathione (GSH) was measured by using the method described by Ellman [11]. Vitamin C was estimated according to the method described by Omaye et al. [12], and Vitamin E was estimated according to the method described by Martinek [13]. Superoxide dismutase (SOD) was estimated according to Flohe and Otting [14], and the activity of catalase was assayed by using the method described by Bisswanger [15].
Descriptive data analysis and evaluation were done by using Sigma Stat 3.1 software. One-way ANOVA was used to determine the differences among groups. The level of significance was calculated using the Turkey test. Values of
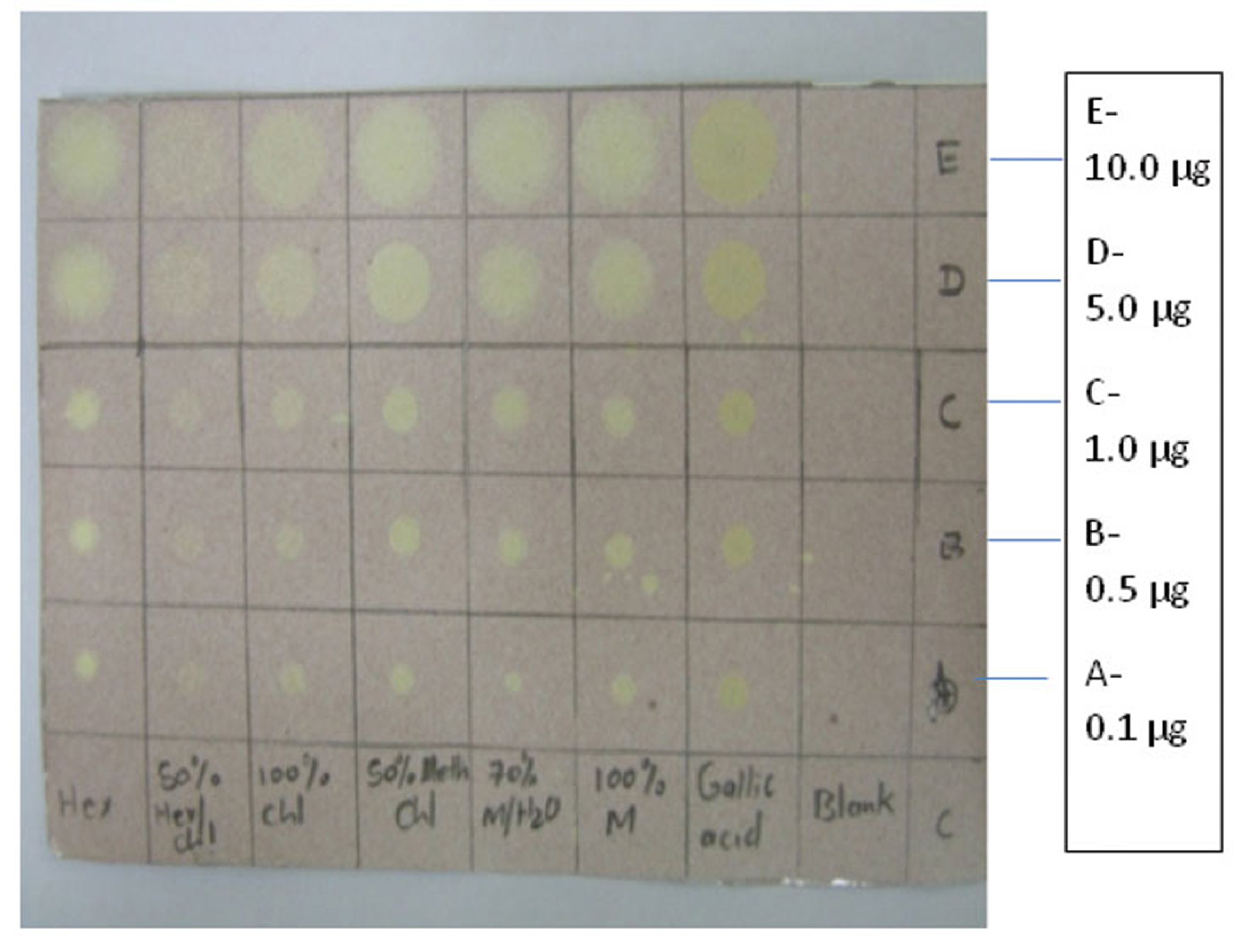
3.1. TLC/DPPH antioxidant activity of the fruit extracts of ZM
Various concentrations (0.1 ㎍, 0.5 ㎍,1.0 ㎍, 5.0 ㎍, and 10.0 ㎍) of the fruit extracts from different solvents spotted on the TLC sheet showed high activity as indicated by the yellow color over the purple DPPH background.
Extracts from 100% methanol, 70% methanol/water, 70% methanol/chloroform,100%chloroform,50% hexane/chloroform and 100% hexane changed to yellow on the purple DPPH background. The extent of yellow coloration was comparable to the gallic acid standard (Fig. 1).
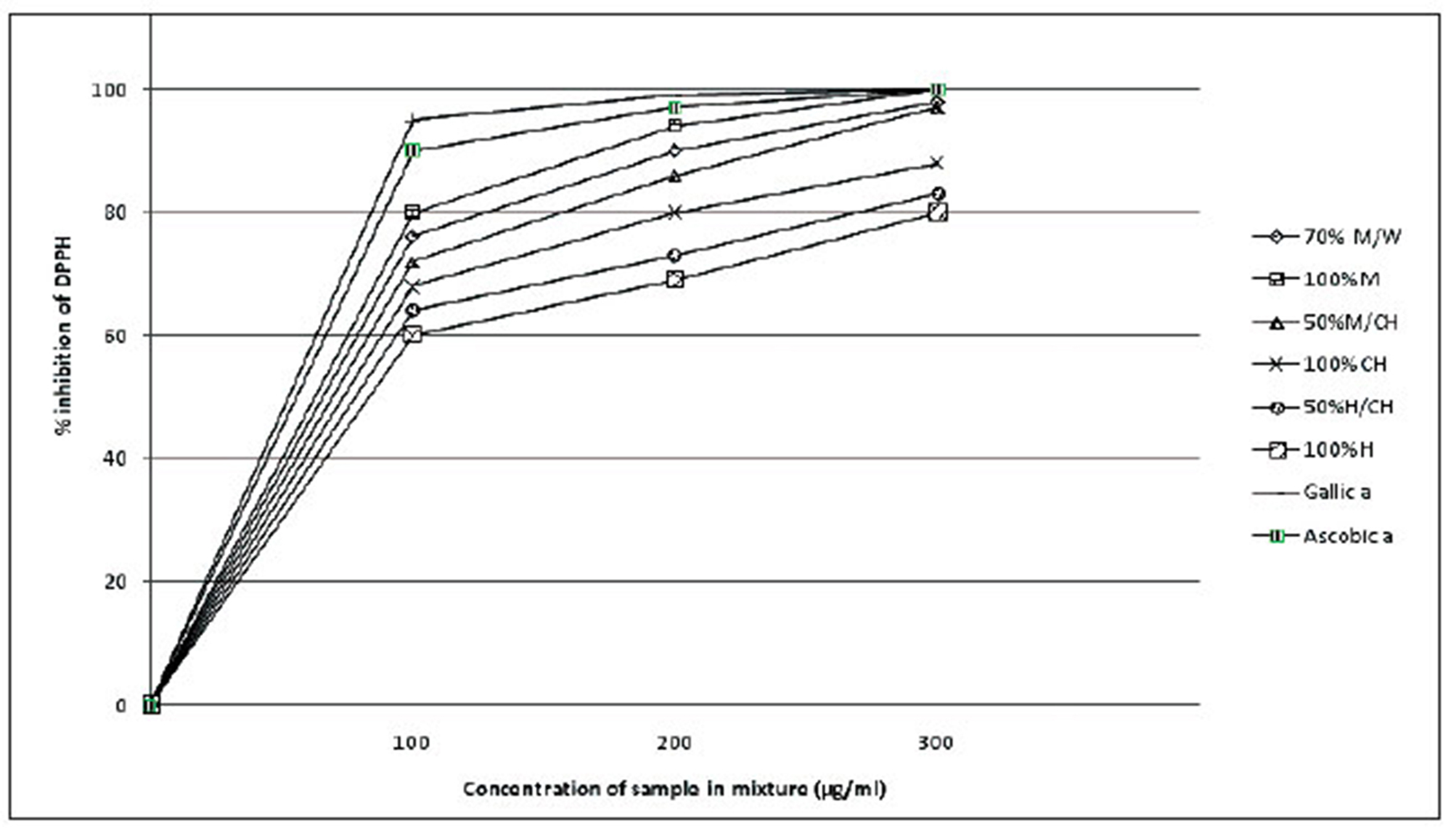
ZM fruit extracts showed high antioxidant activity. The increase in the antioxidant activity occurred in a concentration-dependent manner. The percentage inhibition increased when going from low-polarity-solvent to a high-polarity-solvent extracts: namely, 100% methanol, 70% methanol/ chloroform, 100% chloroform, 50% hexane/chloroform, 100% hexane in that order. The standards, gallic acid, displayed 100% DPPH inhibition (Fig 2).
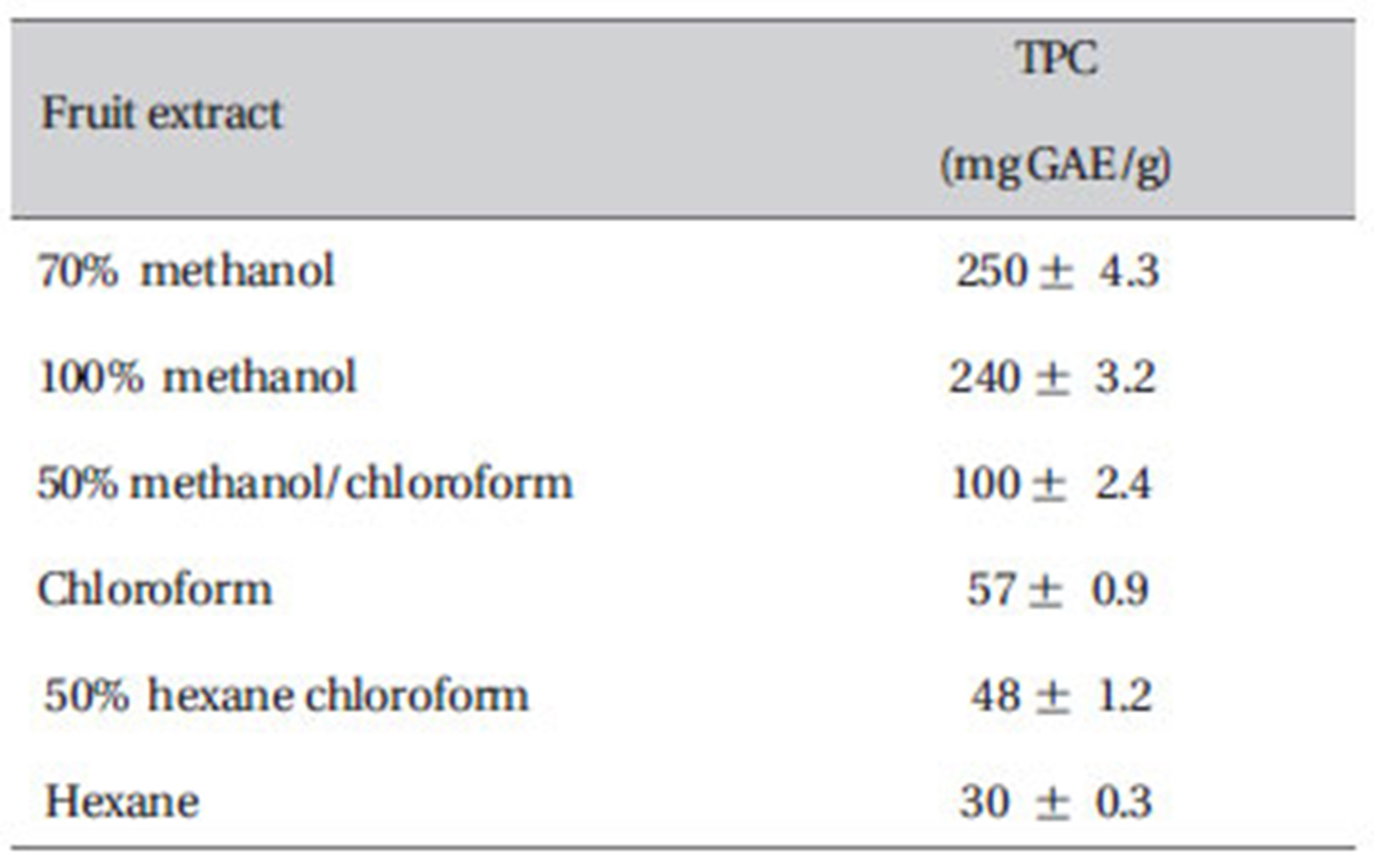
3.3. Total phenol content of different types of fruit extracts of ZM
As presented in Table 1, the phenol content was found to increase from 30 mg GAE/g for hexane extract to 100 mg GAE/g for 50% methanol chloroform, 57 mg GAE/g for chloroform, 48 mg GAE/g for 50% hexane/chloroform and 250 mg GAE/g for 70% methanol extract.
3.4. 3.2.5 In vitro antioxidant activity by ABTS.
Effect of ABTS free radical scavenging activity of
Significant ABTS scavenging activity was evident in 100% methanol extract 7.21, and a weak scavenging activity was observed in 100% hexane (Table 2).
[Table 1] Total phenol content of ZM fruit extracts
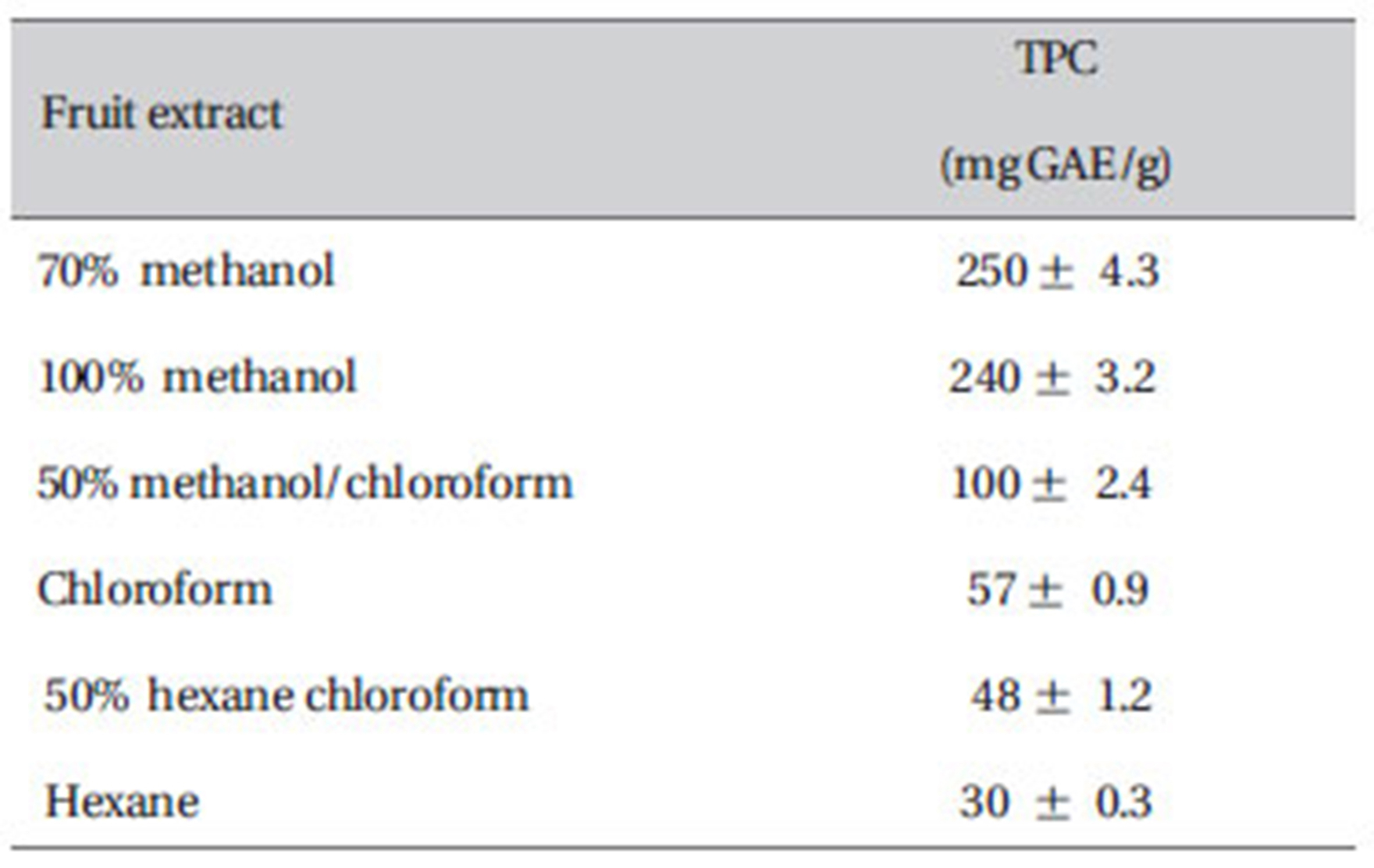
Total phenol content of ZM fruit extracts
[Table 2] In-vitro ABTS free-radical scavenging activity of ZM extracts
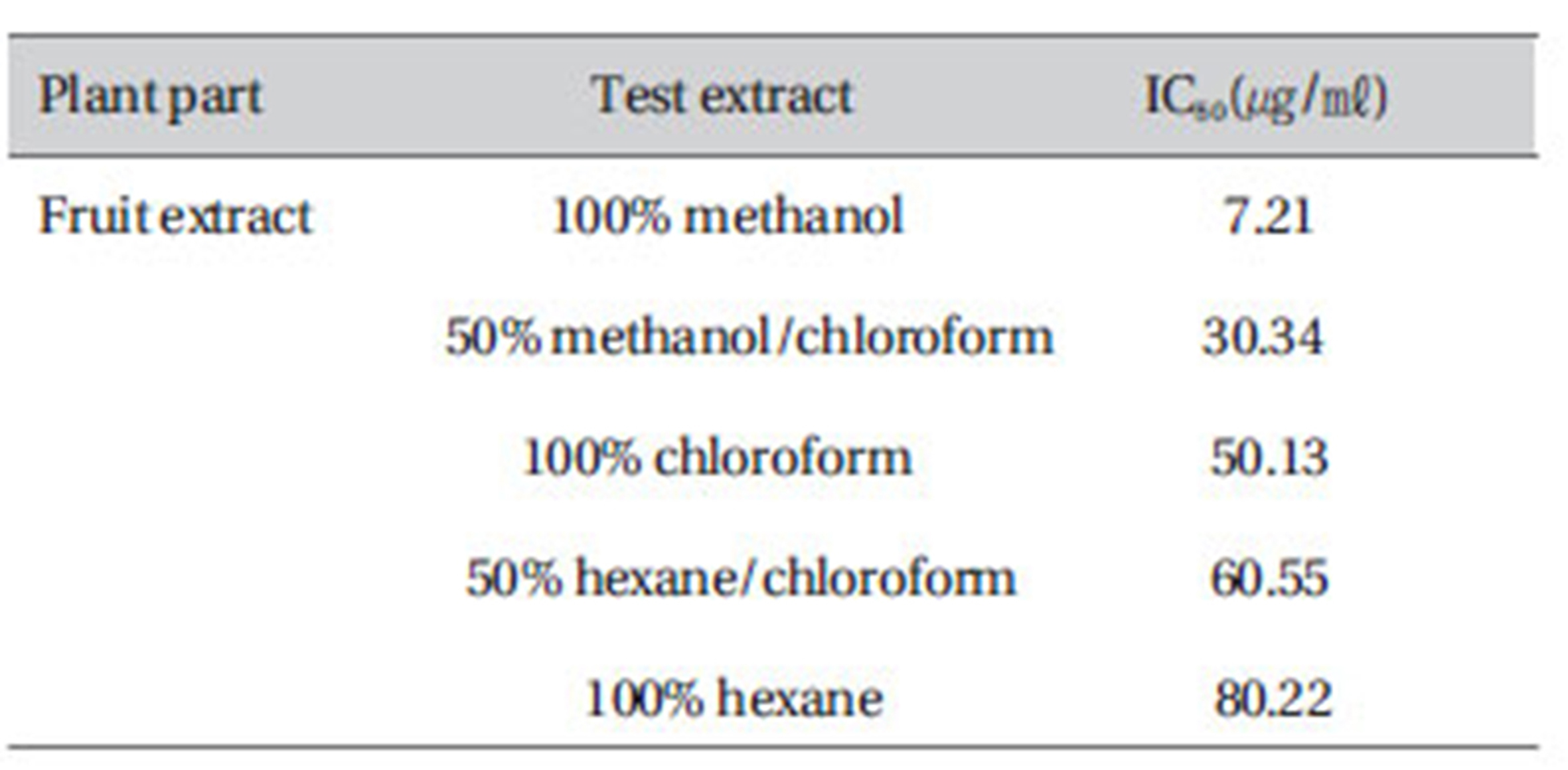
In-vitro ABTS free-radical scavenging activity of ZM extracts
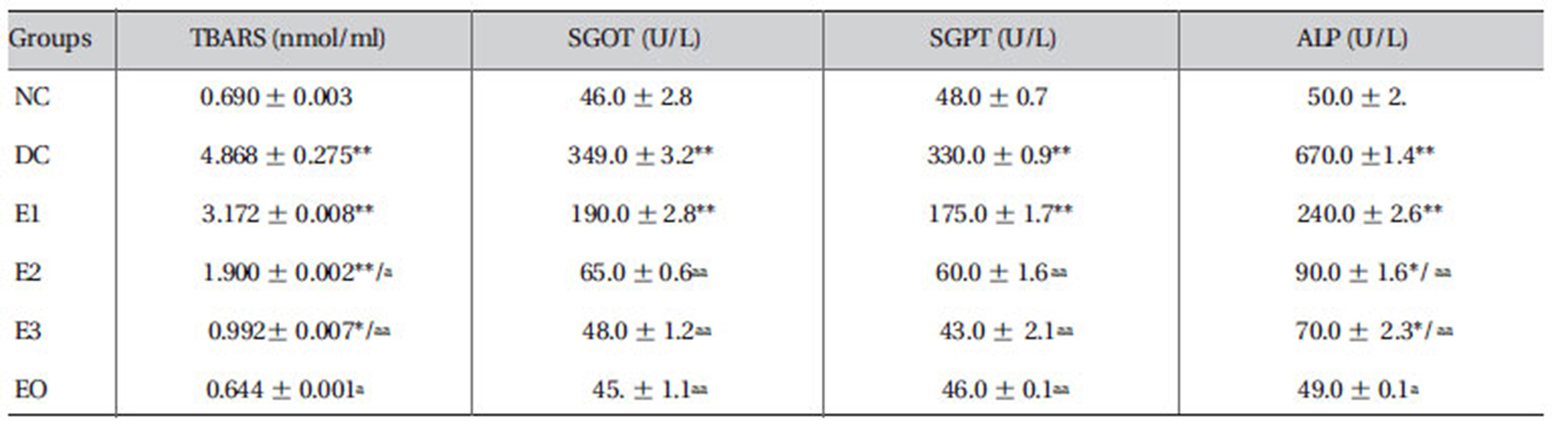
3.5. Effects ZMFM on plasma TBARS and liver transaminases
Daily administration of dimethoate resulted in elevated levels of TBARS in the plasma of the DC group. A dosedependent reduction in the levels of plasma TBARS was associated with the ZMFM administration. Compared with the levels in NC rats, liver transaminases, serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) and alkaline phosphatase (ALP) were increased significantly (
[Table 3] Effect of ZMFM on serum marker parameters of dimethoate-treated rat

Effect of ZMFM on serum marker parameters of dimethoate-treated rat
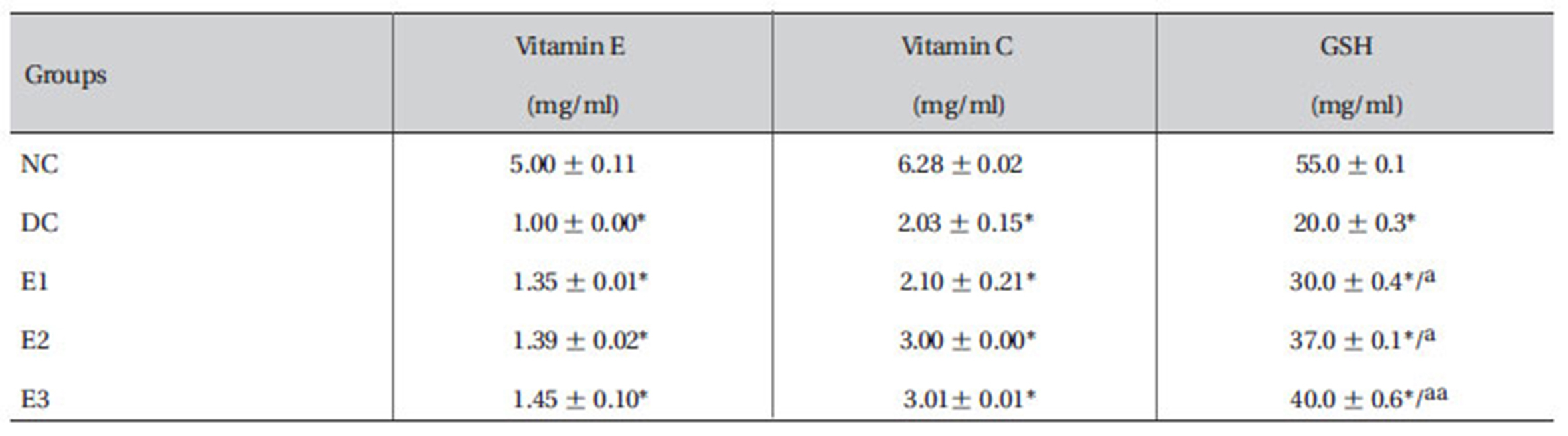
3.6. Effects ZMFM on plasma reduced glutathione (GSH), vitamin C and E.
The results showed that the plasma levels of reduced GSH, vitamin C and E were reduced significantly (
[Table 4] Effect of ZMFM on GSH, vitamin C and vitamin E of dimethoate-treated rats
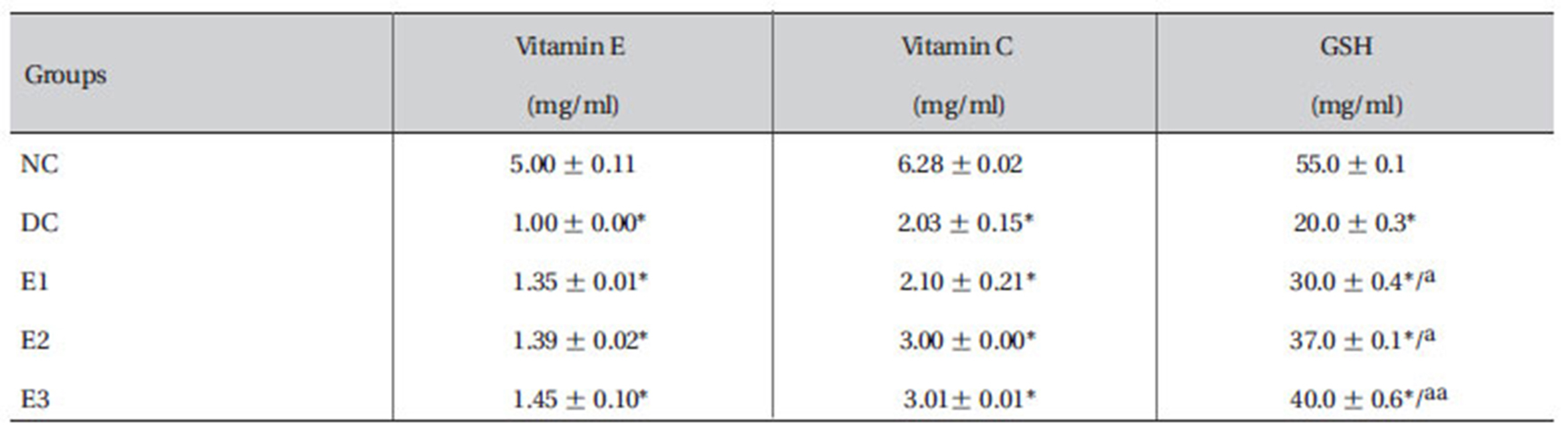
Effect of ZMFM on GSH, vitamin C and vitamin E of dimethoate-treated rats
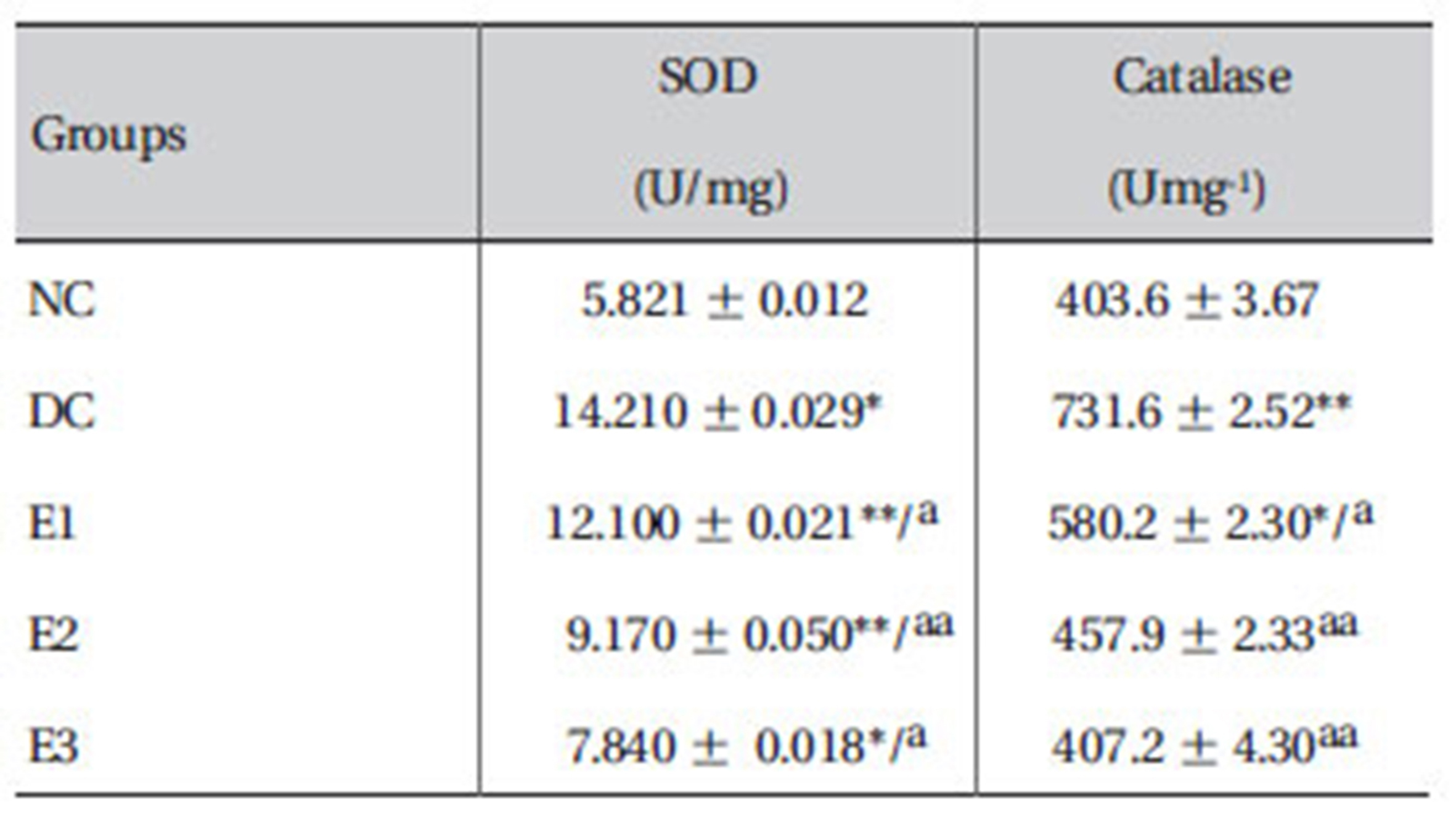
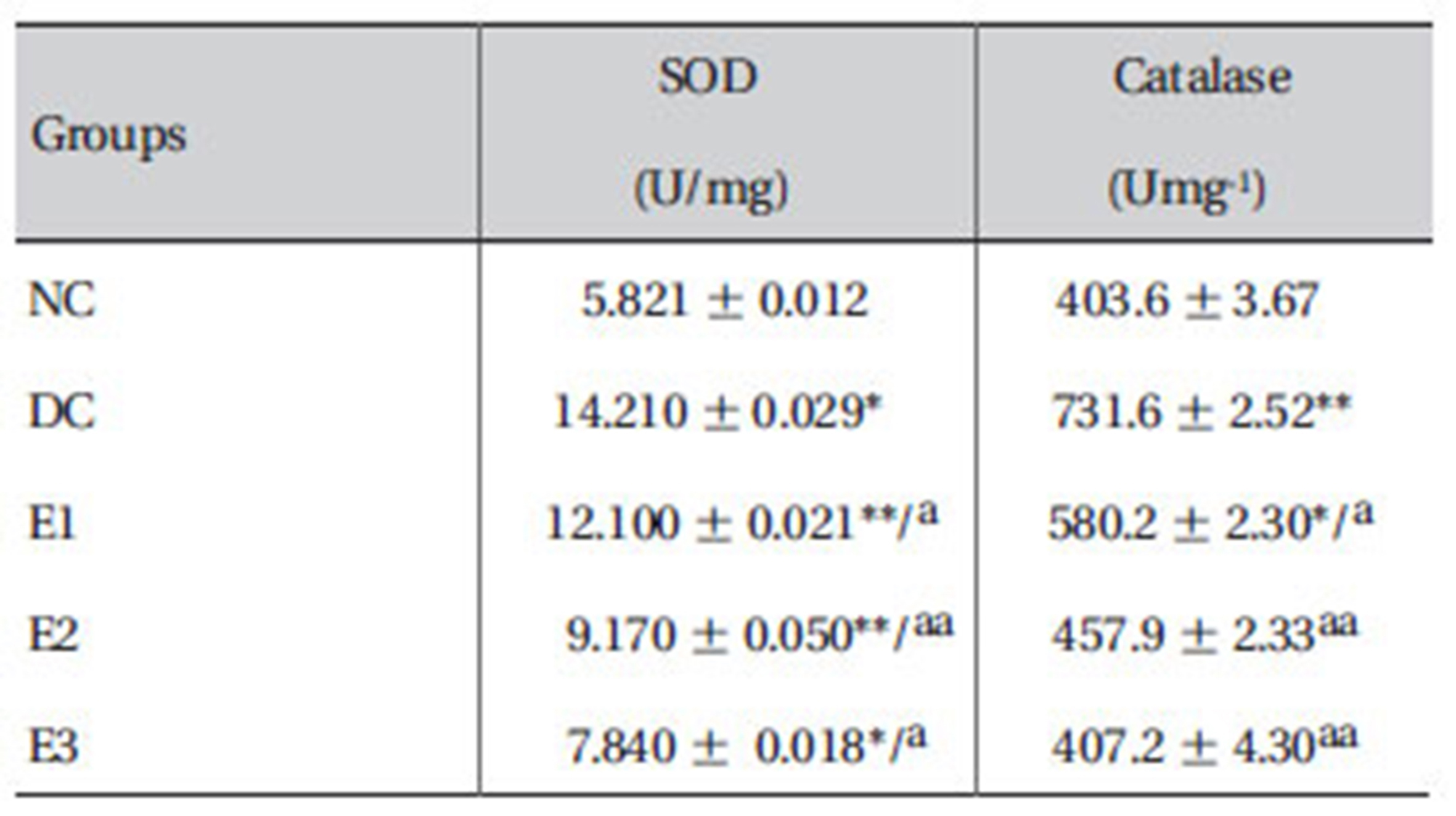
3.7. Effects ZMFM on plasma SOD and catalase activities.
The results showed that the activities of SOD and catalase were enhanced significantly (
Effect of ZMFM on antioxidant enzymes: SOD and catalase in plasma from dimethoate-treated rats
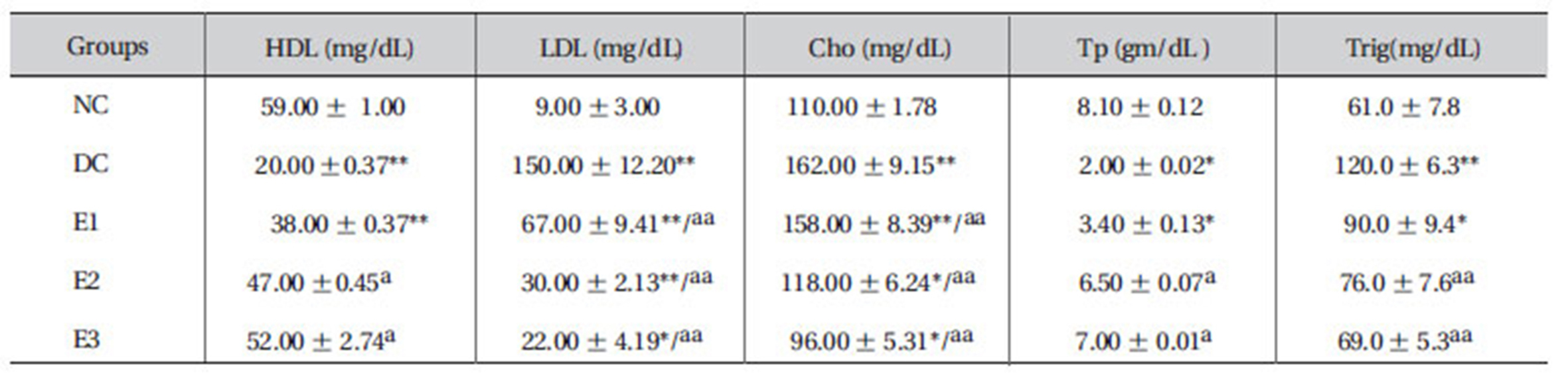
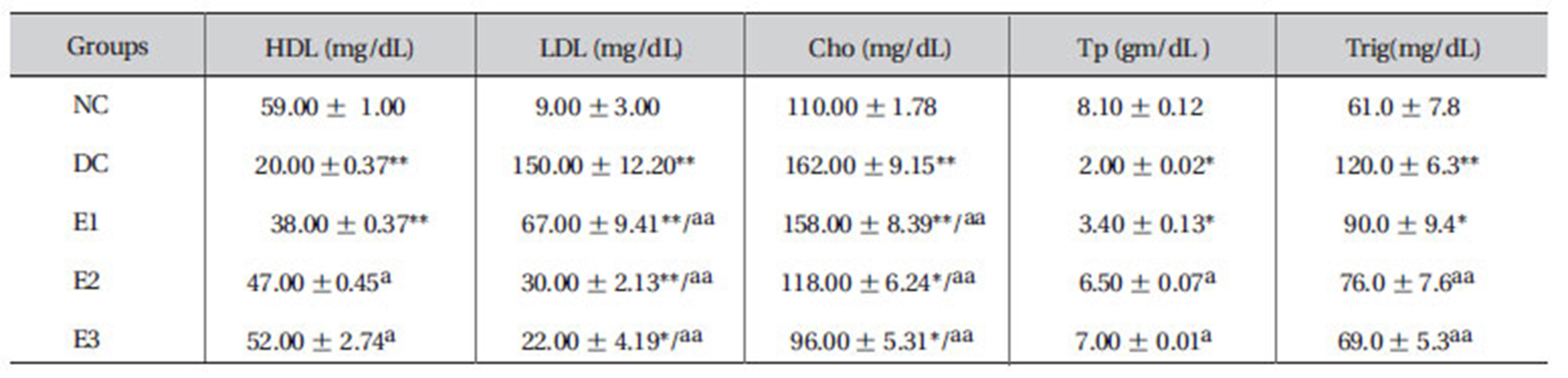
3.8. Effects of ZMFM on lipid profiles and total protein
Dimethoate administration was shown to significantly lower HDL levels (
[Table 6] Effect of ZMFM on lipid and lipoprotein profiles in serum from dimethoate-treated rats
Effect of ZMFM on lipid and lipoprotein profiles in serum from dimethoate-treated rats
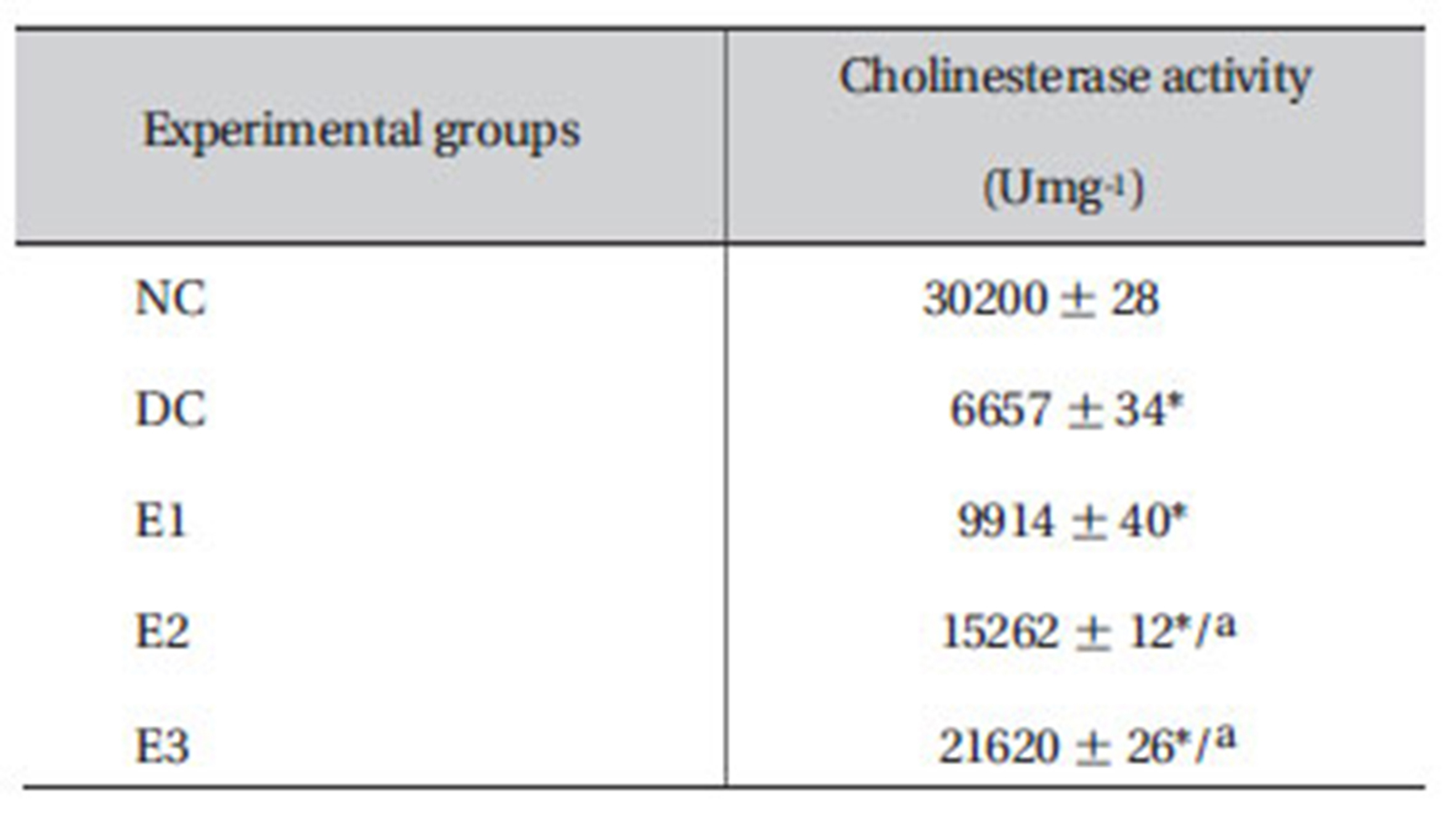
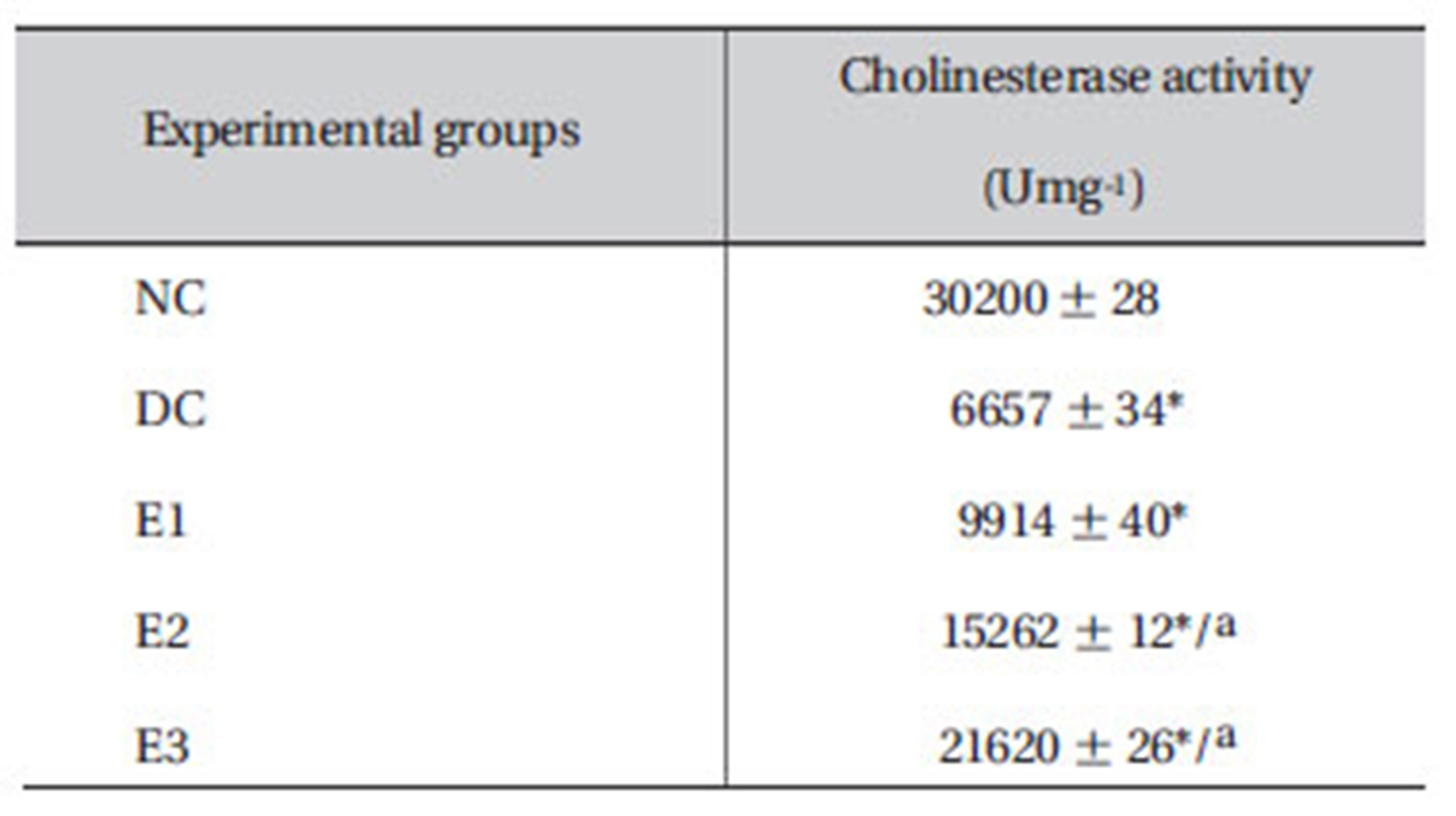
3.9. Effect of ZMFM on cholineasterase activity
The cholinesterase activity declined (
[Table 7] Effects of ZMFM on plasma cholinesterase activity on dim -ethoat-treated rats
Effects of ZMFM on plasma cholinesterase activity on dim -ethoat-treated rats
3.10. Effect of ZMFM on Histology
ZMFM administration revealed a preventive effect on the hepatocytes when compared to the dimethoate control (DC). The DC control group showed evident hepatic damage as shown by widening sinusoids, loss of both hepatocellular membrane and hepatocytes when compared to the normal control group. The preventive effect was more evident with increasing dosage of the ZMFM extract. The preventive effect was shown by reduced necrosis in ZMFM-fed rats when compared to dimethoate-fed rats (Fig. 3).
Exposure to various organic compounds including a number of environmental pollutants and drugs can cause cellular damages through metabolic activation of those compounds by highly reactive substances such as reactive oxygen species (ROS) [17,18]. Dimethoate is one of the organophosphates frequently used in agriculture, and it has been reported to generate oxidative stress and impose toxicity [19].
Organophosphates have been found to inhibit cholinesterase (AchE) and subsequent activation of cholinergic receptors [20]. The use of antioxidants like botanicals is an effective means to fight against the deleterious effects of oxidative stress. Botanicals are natural sources of antioxidants because of their richness in phenol, flavonoids and other anti-oxidants. In the present study, various extracts of ZM were found to contain significant amounts of phenols. In the present study, the total phenol content was seen to increase when going from the less polar solvent extracts to the more polar solvent extracts .The highest concentration was present in the 70% methanol/water extract.
ZM fruit extracts exhibited high antioxidant activity on the TLC/DPPH antioxidant assay (Fig. 1). The TLC-DPPH assay was confirmed by the DPPH spectrophotometric assay (Fig. 2) and ABTS (Table 2) assays. The methanol fruit extract had the highest DPPH scavenging activity (96 shown %)and exhibited 7.21㎍/㎖-Inhibiting concentration (IC50) on ABTS Other solvents showed lower antioxidant activities; hence, the highest antioxidant activity by the methanol extracts best represents the fruits antioxidant potential.
Dimethoate is known to inhibit cholinesterase activity in the target tissues [21]. It accumulates acetylcholine and prevents the smooth transmission of nerve functions, leading to convulsions and death. Dimethoate inhibits the activity of the enzyme cholinesterase that hydrolyses acytylcholine into acetyl and choline and, hence, prevents the return of cholinergic neurons to normal. The liver is the primary organ involved in xenobiotic metabolism and is a major target organ for chemicals and drugs. In the present study, oral administration of dimethoate to rats caused hepatic damage, as evidenced by the elevations in the hepatospecific enzyme activities, as well as by the severe alterations in different liver marker parameters. TBARS, SGOT, SGPT and ALP were significantly increased (
The levels of GSH, vitamin C and vitamin E were observed to decline (
Vitamin E is thought to be an important chain-breaking antioxidant, is the major soluble antioxidant in all cellular membranes, and protects against lipid peroxidation [23]. ZMFM may possibly play a role in scavenging the reactive oxygen species (ROS). This thus allows the recovery of vitamin E which was used up in scavenging dimethoateinduced radicals.
The observed significant (
The observed elevated catalase activity may be a result of an adaptive response to the generated free radicals [3]. This directly indicates the failure of the total antioxidant defense system as shown by lipid peroxidation. The superoxide ion generated is, therefore, accounted for by the enhanced SOD and is converted to H2O2 or glutathione peroxidase. A decrease in the catalase activity suggests the possibility that ZMFM scavenges the free radicals before they necessitate a natural adaptive response by the enzyme. The study, thus suggests the ability of ZMFM to alleviate dimethoate-induced oxidative stress.
Elevated low-density lipoprotein (LDL), cholesterol (Cho) and triglyceride (Trig) levels and decreased high-density lipoprotein (HDL) and total protein levels were observed in dimethoate-treated rats, when compared to normal control rats, after 90 days of treatment. Administration of ZMFM extract resulted in significant (
LDLs are known to carry cholesterol to the peripheral tissues where it can be deposited and increase the risk of atherosclerotic attack and peripheral vascular disease. LDL accumulation is, thus, very bad for the body [24]. The orally-administered extract offers more benefits by increasing the level of HDLs in rat plasma. Unlike the LDLs, HDLs hasten the removal of cholesterol from peripheral tissues to the liver for catabolism and excretion [1,25].
The histological studies of the liver sections presented in Fig. 3 provide supportive evidence for the protective effects of ZMFM. The liver sections of normal control animals showed normal hepatic cells, each with preserved and prominent hepatocytes. The liver sections of dimethoateintoxicated rats showed loss of hepatocytes and loss of cellular boundaries and widened sinusoids; ZMFM attenuated the degenerative effects of dimethoate toxicity. This was observed in the reduced intensity in liver-tissue degenerative changes. Here, less necrosis, limited loss of cell boundaries and reduced loss of intact hepatocytes were observed. The preventive effect of the extracts increased with increasing dosage of the extracts.
In conclusion, ZMFM extract could protect against oxidative stress by decreasing lipid peroxidation and enhancing the effects of enzymatic and non-enzymatic antioxidants. It also maintained the lipid profile and protected the liver from oxidative damage. These protective effects are possibly because of the high antioxidant potential of ZMFM.