Mast cells are key effector cells in IgE-associated immune responses, including allergic disorders such as anaphylaxis, atopic dermatitis, allergic rhinitis, and allergic asthma. Mast cell activation through FcεRI and preformed and newly synthesized mediator release results in allergic inflammatory conditions: erythema, edema, and itching in the skin; sneezing, rhinorrhea, cough, bronchospasm, and mucous secretion in the respiratory tract; and nausea, vomiting, diarrhea, and cramping in the gastrointestinal tract [1].
Interleukin (IL)-1 is a major proinflammatory cytokine that induces acute and chronic inflammation through activation of the innate and the acquired immune systems. IL-1 can enhance cytokine secretion [2-5] and histamine [6] in the development of IgE/Ag-mediated allergic diseases such as allergic asthma. IL-6, a multifunctional cytokine, is produced by a variety of hematopoietic and non-hematopoietic cell types in response to diverse stimuli [7]. Recent studies have shown that IL-6 plays a role in the development and the exacerbation of Th2-mediated diseases such as allergic airway inflammation and asthma [8]. Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been implicated as an important mediator in the pathogenesis of asthma [9]. In particular GM-CSF is pivotal in eosinophil maturation and survival [10], a key effector cell in asthma.
Inflammatory mediators such as histamine, leukotrienes and prostaglandins are synthesized by enzymes such as L-histidine decarboxylase (HDC), cyclooxygenase (COX)-1, COX-2 and 5-lipoxygenase (LO) in mast cells activated with IgE/antigen (Ag). As HDC is the sole enzyme that produces histamine by decarboxylation of L-histidine in mammals, HDC is an important regulator of histamine signaling [11]. COX contributes to the synthesis of prostaglandins, such as prostaglandin D2 (PGD2) and prostaglandin E2 (PGE2), which are inflammatory lipid mediators from arachidonic acid. COX-1 is associated with the immediate PGE2 and PGD2 generation and COX-2 is associated with the delayed PGD2 generation [12-14] 5-LO synthesizes leukotriene, another class of inflammatory lipid mediators from arachidonic acid [15].
Mast cells express a high-affinity receptor for IgE (FcεRI) that binds to Ag-specific IgE on the cell surface. FcεRI consists of an IgE binding
Phosphorylated Syk leads to downstream signaling with phosphorylation of linker for activation of T cells (LAT). LAT functions as a signal platform providing binding sites for phospholipase C
The root of Rehmannia glutinosa is mainly used for Yin deficiency syndrome in traditional medicine in the East Asian region. It has been frequently used to reduce inflammation and is included in various formulas [21-25]. It inhibits inflammation in the development of atopic dermatitis [25] and in the diabetic foot ulcer rat model [23]. The objective of the present study was to examine the proinflammatory cytokines and enzymes responsible for the anti-allergic inflammatory activity of RGPS. In addition, the underlying anti-allergic inflammatory mechanism of RGPS was investigated.
RGPS was purified from the roots of Rehmannia glutinosa. The preparation of ethanol extracts of Rehmannia glutinosa took place at Dong-Eui University Oriental Medicine Hospital. The roots of Rehmannia glutinosa (800 g) were extracted with 25% ethanol (4 L) for 10 h at room temperature. The extracted solution (RGPS) was filtrated and concentrated to a 400-ml ethanol extract. RGPS was diluted in Dulbeco's Modified Eagle's Medium (DMEM) media containing ethanol, and the final concentration of ethanol was adjusted to 1% (v/v) in the cell culture system. The control cells were treated with media containing 1% ethanol.
Rat basophilic leukemia RBL-2H3 cells were obtained from the Korea Cell Line Bank (Seoul, Korea). The cell line was cultured in DMEM supplemented with 10% heatinactivated fetal bovine serum (FBS) and 100 U/ml of penicillin and streptomycin in an atmosphere of 5% CO2 at 37℃. Cells were detached with trypsin-EDTA solution. After the cells had been washed, they were resuspended in fresh medium and used for subsequent experiments.
Chemicals and cell culture materials were obtained from the following sources: anti-dinitrophenyl (DNP) IgE, DNP-human serum albumin (HSA),
RBL-2H3 cells (5 × 105 cells/ml) were sensitized with anti-DNP IgE (0.5
2.5. RNA preparation and RT-PCR
RBL-2H3 cells (1 × 106 cells/ml) were sensitized with anti DNP-IgE overnight. The cells were pretreated with or without RGPS for 1 h and were then stimulated with DNP-HSA for 4 h at 37℃ in 5% CO2. The cells were then chilled with ice to terminate the stimulation. Thereafter, the cells were washed twice with ice-cold PBS; then, the total RNA was extracted from the cells by using TRIzol reagent according to the manufacturer’s instructions. The PCR products were electrophoresed in 2% (w/v) agarose gels and were stained with ethidium bromide (EtBr) (Amresco). The detection and the densitometric analyses of the bands were performed with a Scion Image System (Scion Corporation). The sizes of bands were confirmed with reference to molecular size markers (100 bp DNA Ladder Marker, Invitrogen). The amount of mRNA for each cytokine was normalized to the amount of GAPDH mRNA, which was utilized as a housekeeping gene for each experimental condition
2.6. Protein preparation and western blotting
RBL-2H3 cells (1 × 106 cells/ml) were sensitized with anti DNP-IgE overnight. The cells were pretreated with or without RGPS for 1 h and were then stimulated with DNP-HSA for 15 min at 37℃ in 5% CO2. The cells were then chilled with ice to terminate the stimulation. Thereafter, the cells were washed twice with ice-cold PBS and lysed in 0.5 ml with an ice-cold lysis buffer (20 mmol/L HEPES [pH 7.9), 0.4 mmol/L NaCl, 1% Igepal CA-630, 10% glycerol, 5 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L DTT, 1 mmol/L EDTA, 1 mmol/L EGTA, and 0.5 mmol/L PMSF). The lysates were kept on ice for 30 min, followed by centrifugation at 15,000 g for 15 min at 4℃. The proteins were separated by using sodium dodecyl sulfatepolyacrylamide gel electrophoresis with 8% polyacrylamide gels and were transferred to nitrocellulose transfer membranes (Whatman, GmbH). Subsequent to blocking in a TBS-T buffer (10 mmol/L Tris-HCl ]undefined [pH7.5], 150 mmol/L NaCl, and 0.05% Tween 20) containing 5% skimmed milk powder, the membrane was incubated with individual antibodies. The primary antibodies were diluted 1:1000-fold unless otherwise noted and were incubated at 4℃ overnight. The membranes were washed 3 times for 5 min each with the TBS-T buffer. The immunoreactive proteins were incubated using horseradish peroxidase-coupled secondary antibodies diluted 1:2000-fold for 1 h at room temperature, were subsequently washed 3 times (10 min each wash) with the TBS-T buffer, and were developed with enhanced chemoluminescence, according to the manufacturer’s protocols (Amersham Biosciences, Piscataway, NJ).
Data are presented as means ± standard deviations (SDs). The data were evaluated by using the one way analysis of variance (ANOVA) followed by the least significant difference. Differences among groups were analyzed using Dunnett’s test, and those at
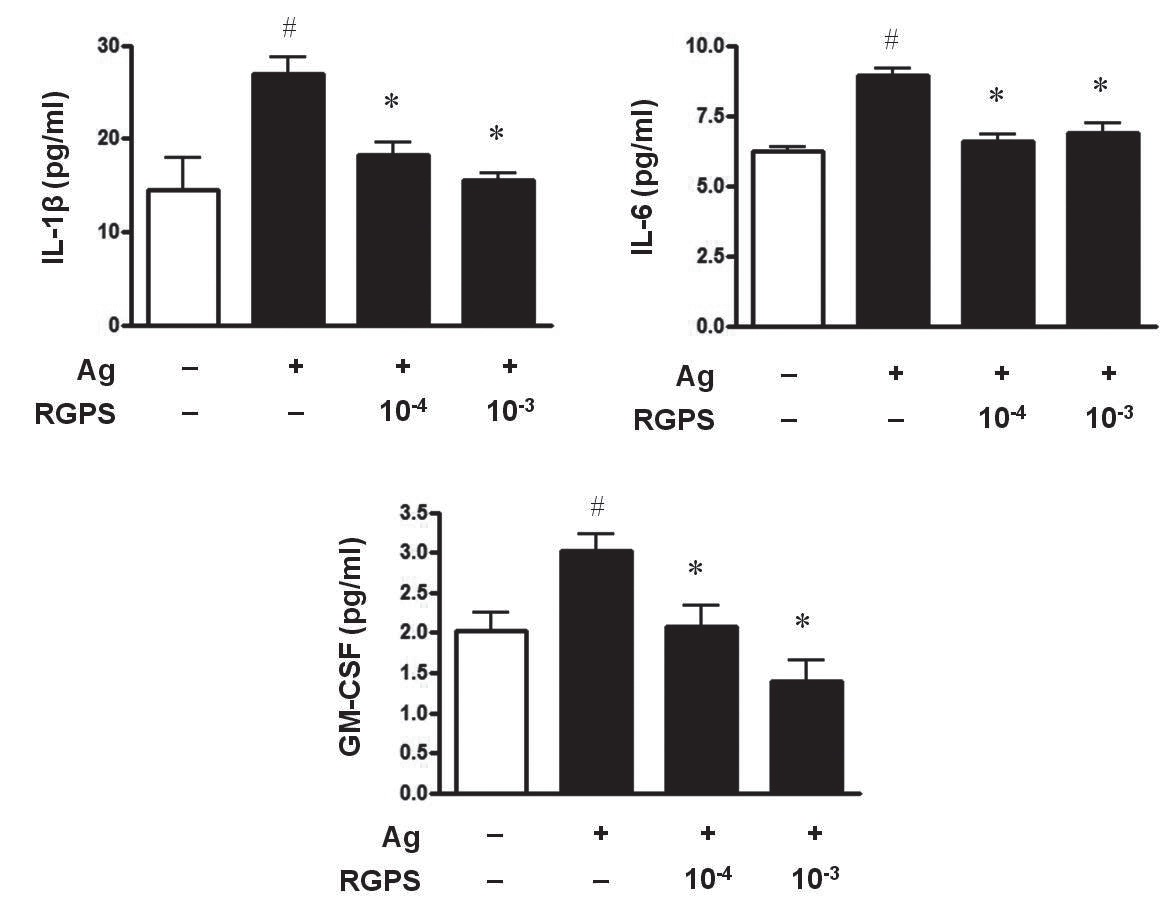
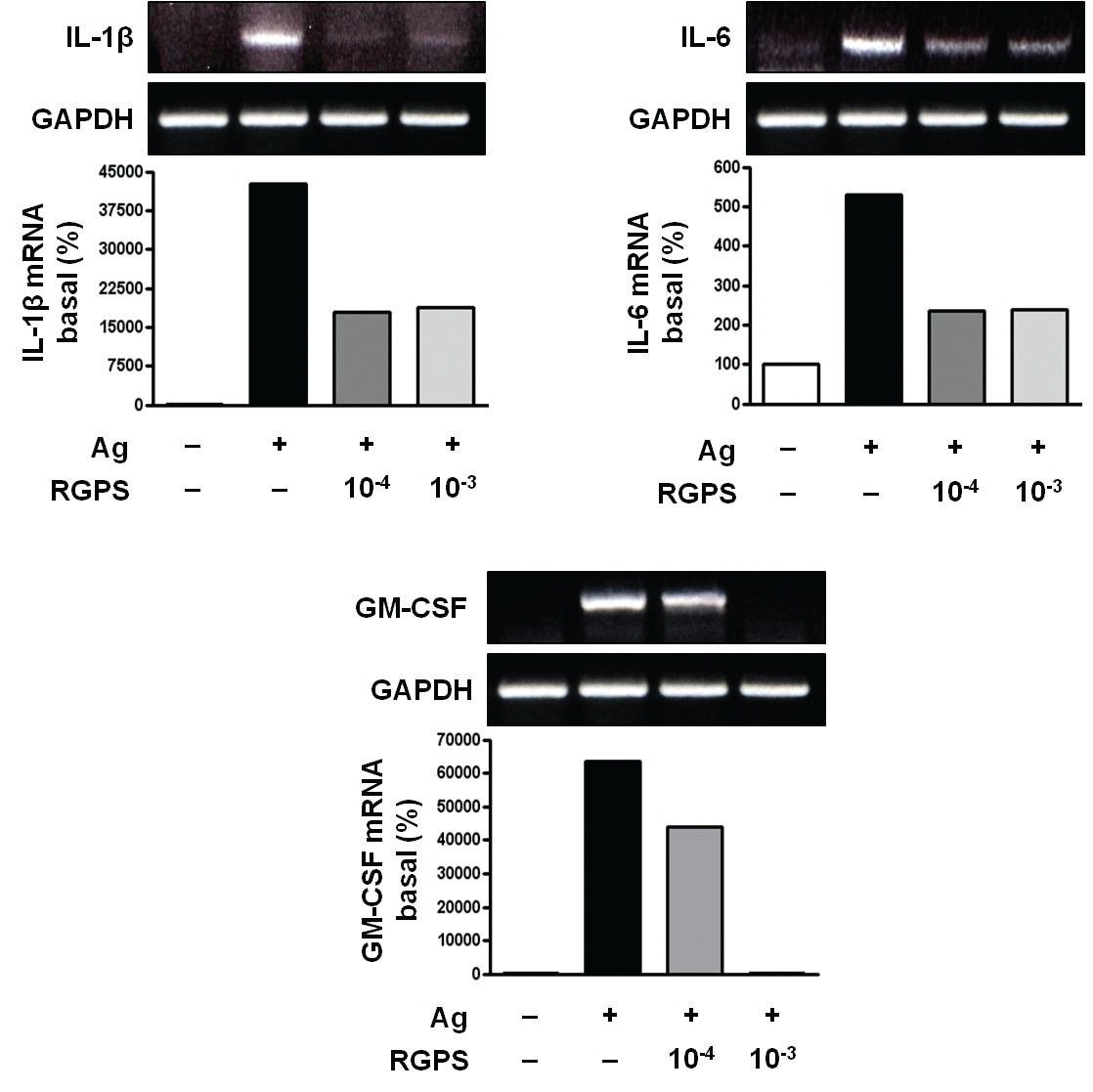
3.1. RGPS suppresses proinflammatory cytokines, IL-1β, IL-6, and GM-CSF
To determine whether RGPS suppresses the secretion of proinflammatory cytokines, IL-1
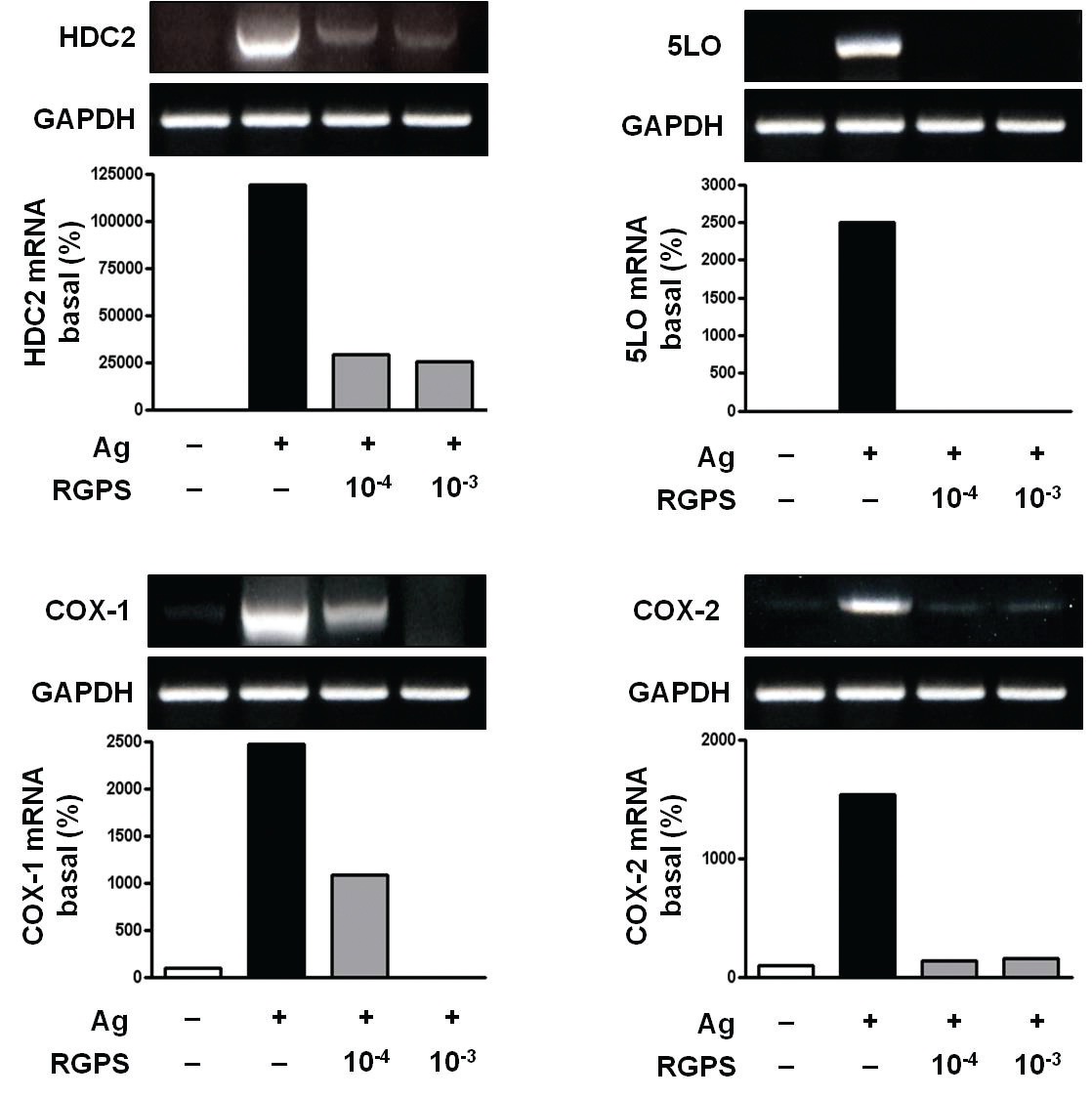
3.2. RGPS suppresses induction of inflammatory enzymes
Inflammatory enzymes, such as HDC2, COX-1, COX-2 and 5LO mRNA, are involved in the synthesis of typical allergic mediators, such as histamine, prostaglandins and leukotriene. Thus, we measured whether RGPS suppresses the induction of these genes by using RT-PCR. Compared to the basal levels of HDC2, COX-1, COX-2 and 5LO
mRNA, the induction levels of those enzymes in IgE-sensitized RBL-2H3 cells was markedly increased after Ag stimulation. RGPS reduced induction of those enzyme genes by Ag stimulation (Fig. 3).
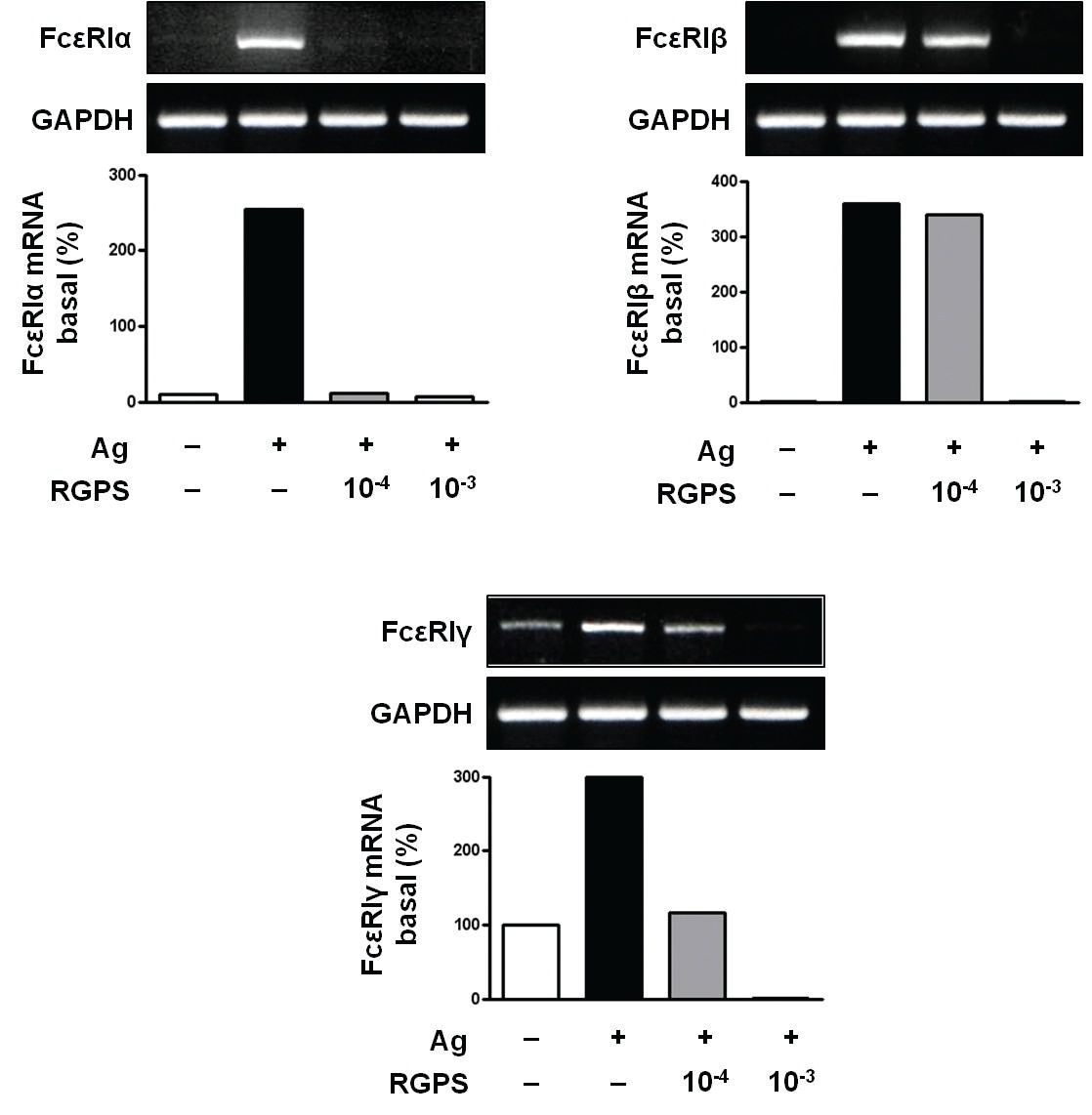
3.3. RGPS suppresses the gene expressions of FcεRIα, FcεRIβ and FcεRIγ
Recent studies have shown that receptors with bound IgE appears to be permanently expressed on the surface of the cells while empty receptors are internalized and degraded [26]. Thus, the density of FcεRI expression correlates with the IgE level, where binding of IgE stabilizes the receptor at the cell surface. Compared to the basal levels of FcεRI
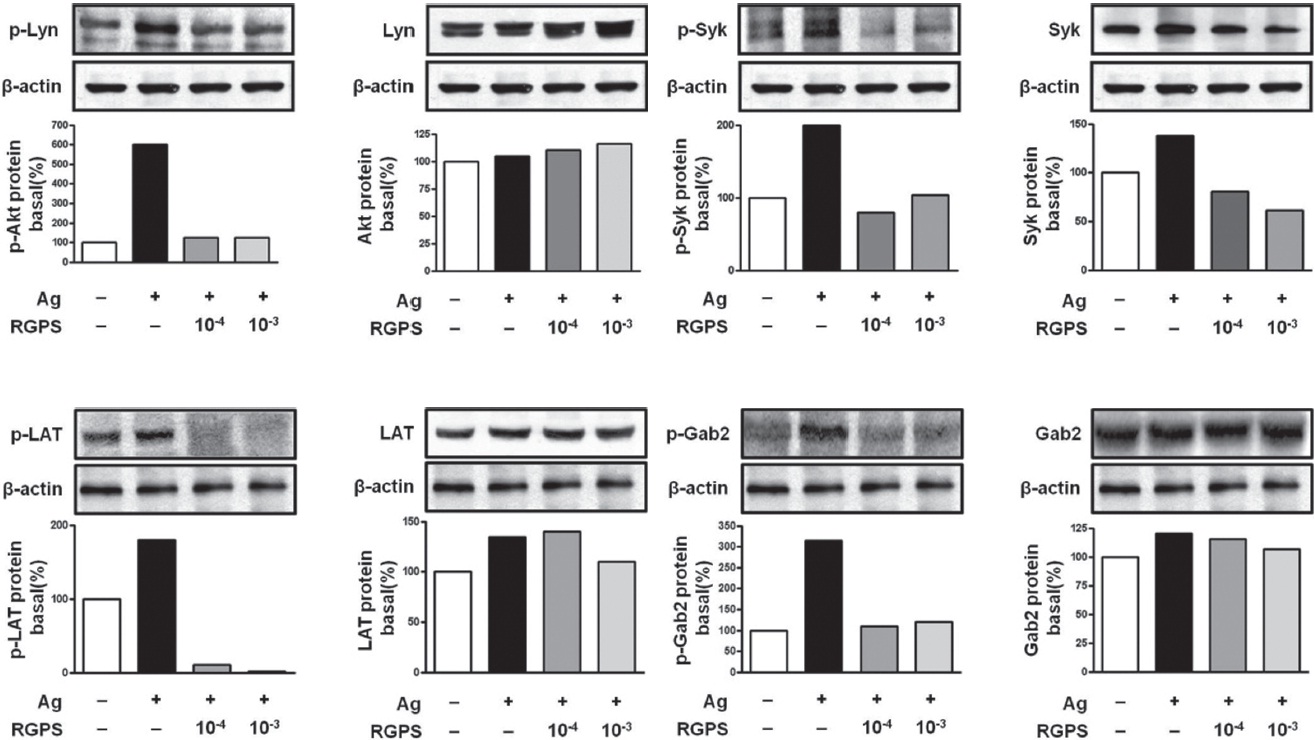
3.4. RGPS suppresses FcεRI-mediated signaling events in IgE/Ag-stimulated mast cells
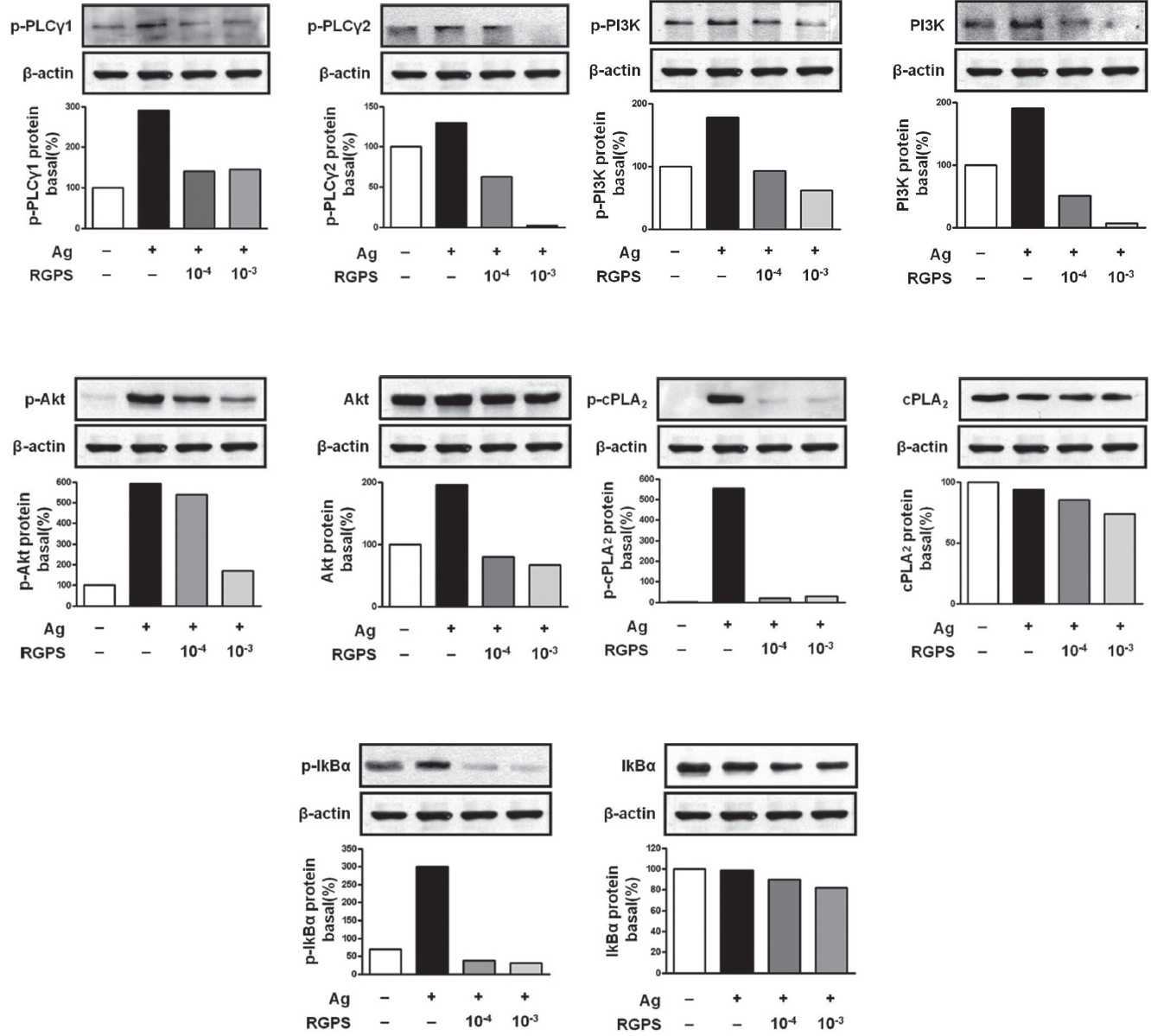
To understand the mechanism for the inhibitory effect of mast cell activation by RGPS, we examined its effects on FcεRI-mediated signaling events. IgE/Ag-mediated FcεRI aggregation induces activation of a Src family kinase, Lyn, which phosphorylates Syk and LAT, or a second Src family
kinase, Fyn, which phosphorylates Gab2. We found that RGPS suppressed Ag-induced phospho-activation of Lyn, Syk, LAT and Gab2 in proximal signaling events of IgE/Agstimulated mast cells (Fig. 5). The adaptor molecule LAT regulates activation of the PLC
We already demonstrated in previous studies that RGPS could suppress cytokine production via MAPKs and NF-
RGPS suppressed the production and gene expression of proinflammatory cytokines and the gene expression of enzymes associated with the production of histamine, prostaglandins and leukotrienes (Figs. 1,2 and 3). The results indicate that RGPS can effectively regulate functional activations, such as degranulation and inflammatory response triggered by cytokines and lipid mediators, in IgE/Ag-stimulated mast cells.
RGPS suppressed the gene expression of FcεRI
The FcεRI signaling event is thought to be mediated by at least two major signaling pathways [18]. First, FcεRI aggregation activates Lyn, which mediates ITAM phosphorylation of the
In summary, RGPS down-regulates both the gene
expression of FcεRI