The order Stichotrichida was established by Faure-Fremiet (1961) on the bases of having a generally ellipsoidal body shape, being small to large in size, and having one or more longitudinally-arranged ventral cirral row. This order includes six families (Corliss, 1979; Berger, 1999, 2006, 2008, 2011; Lynn and Small, 2002; Lynn, 2008). The two stichotrichs in present study belong to the families Amphisiellidae and Kahliellidae. These two families are quite different from each other in their morphology and morphogenesis. The members of Amphisiellidae have a single ventral cirral row formed by two segments comprising ventral anlage IV and V, whereas more than two ventral cirral rows and neokinetal anlage are present in those of Kahliellidae. The amphisiellid genus
>
Sample collection and enrichment
Ciliates samples were collected from marine waters in the tidal pool on the basal rock in Gwangchigi coast (33°27′04′′ N, 126°55′28′′E) located in Jeju Island:
The morphology of the living specimens was examined using a light microscope equipped with a DIC device (Axio Imager A1; Carl Zeiss, Oberkochen, Germany) at low (50-400×) and high (1,000×; immersion oil) magnifications. The images of specimens were captured by a CCD camera (Axio Cam MRc; Carl Zeiss). The infraciliatures were examined after protargol impregnations (Wilbert, 1975; Foissner, 1992). The terminology and the taxonomic classification used follow the guidelines given by Berger (2008, 2011) and Lynn (2008).
Order Stichotrichida Faure-Fremiet, 1961
Family Amphisiellidae Jankowski, 1979
1*Genus Afroamphisiella Foissner, Agatha and Berger, 2002
2*Afroamphisiella multinucleata Foissner, Agatha and Berger, 2002 (Tables 1, 2, Figs. 1-3)
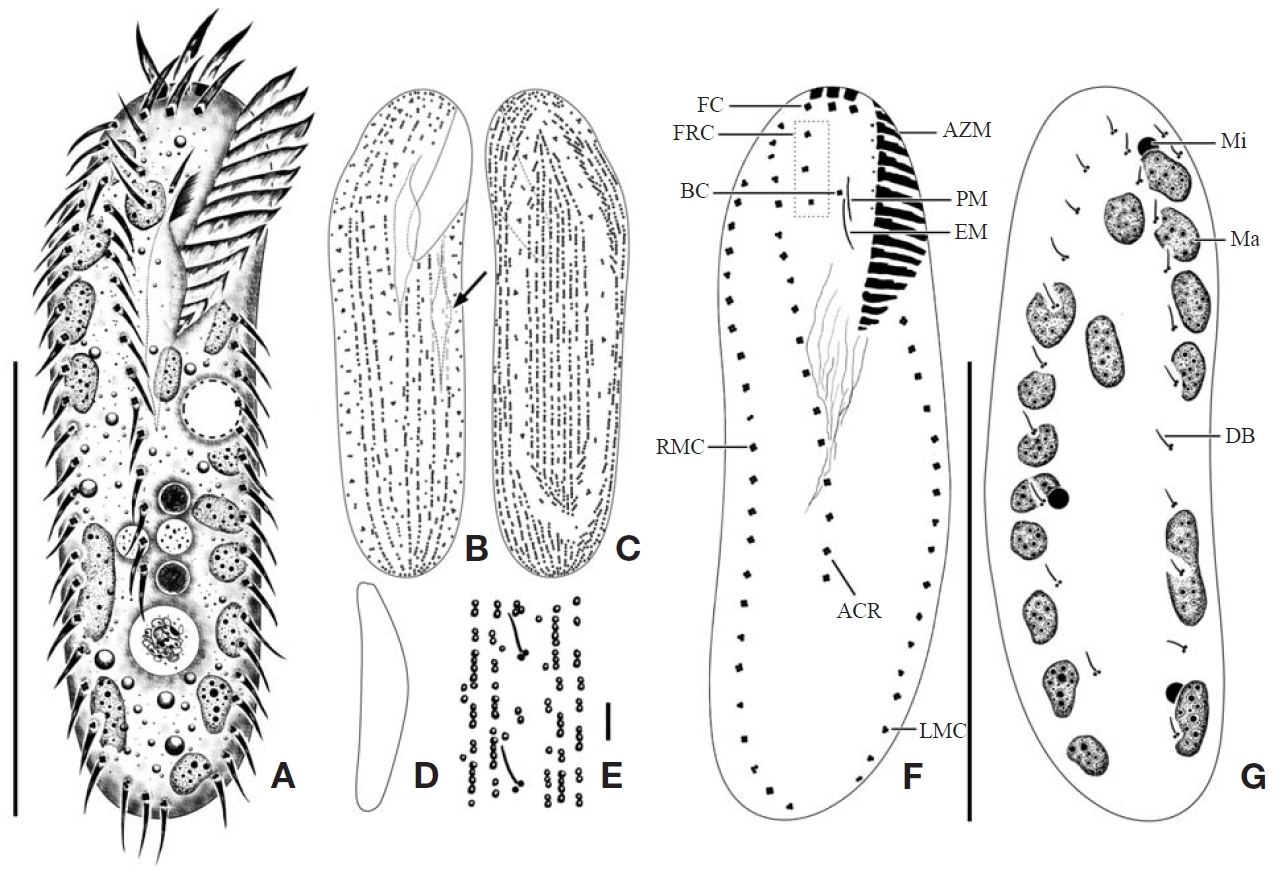
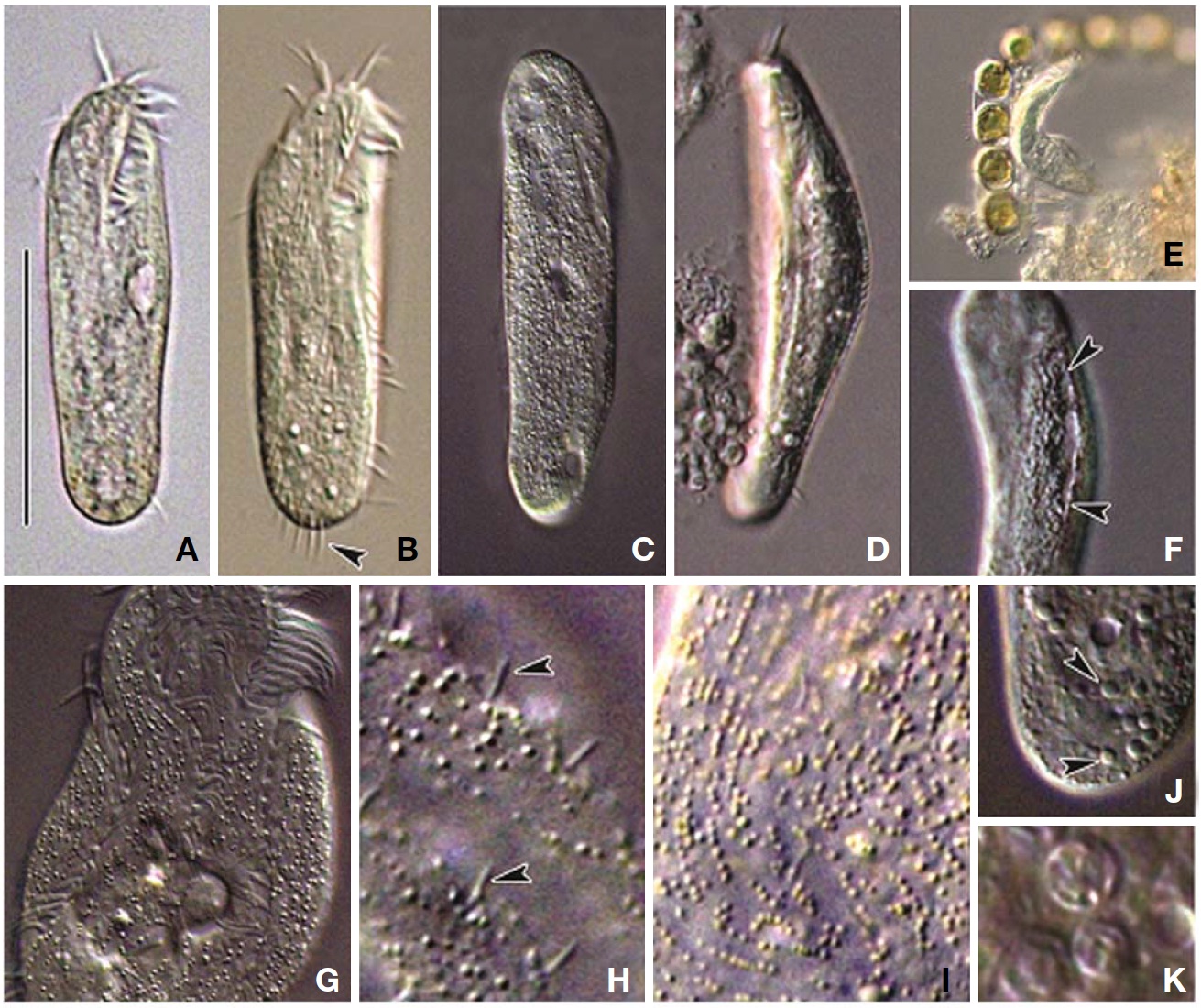
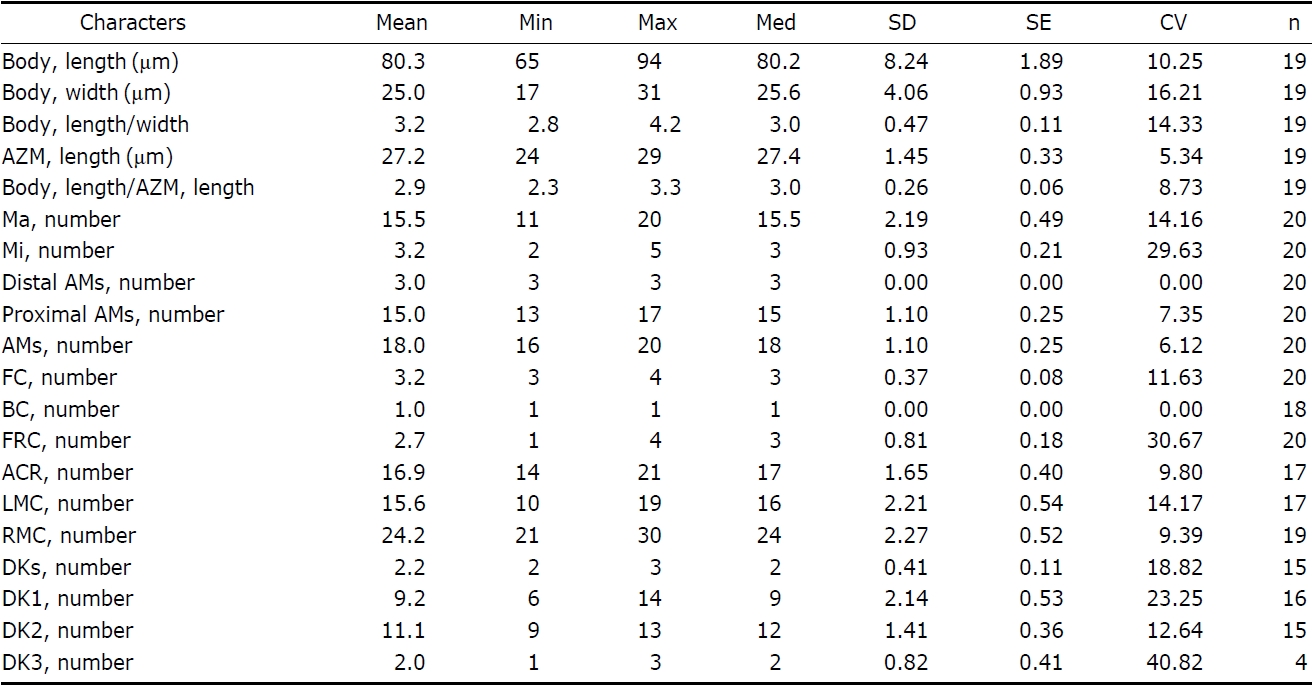
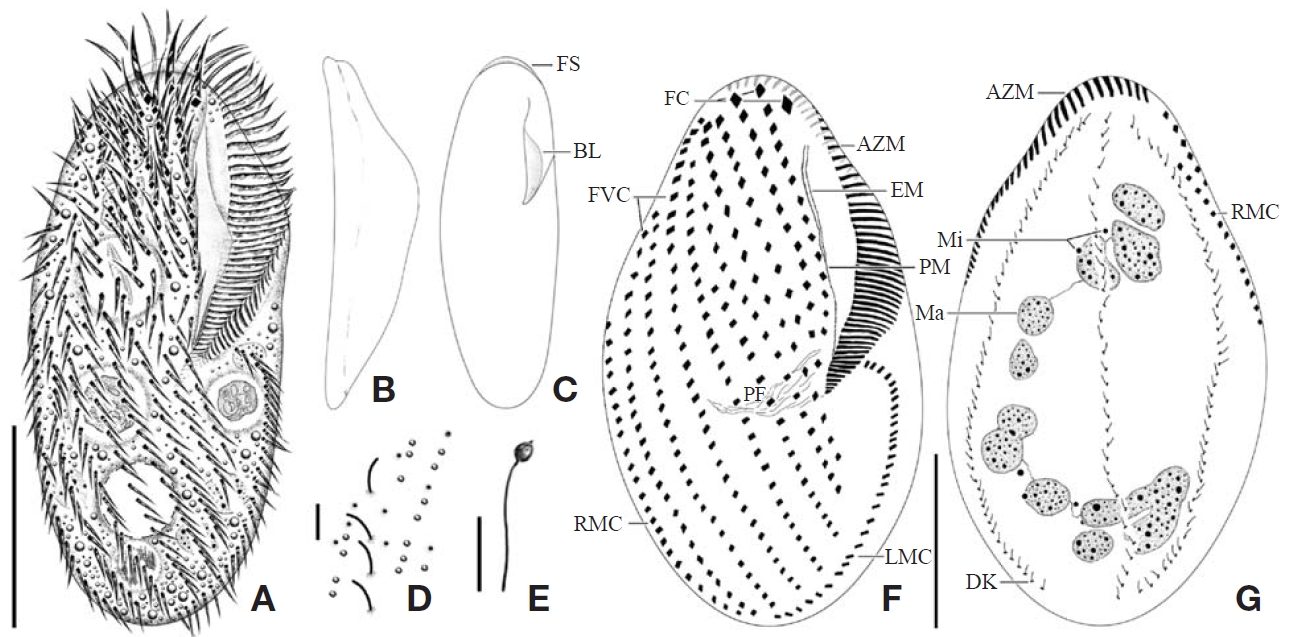
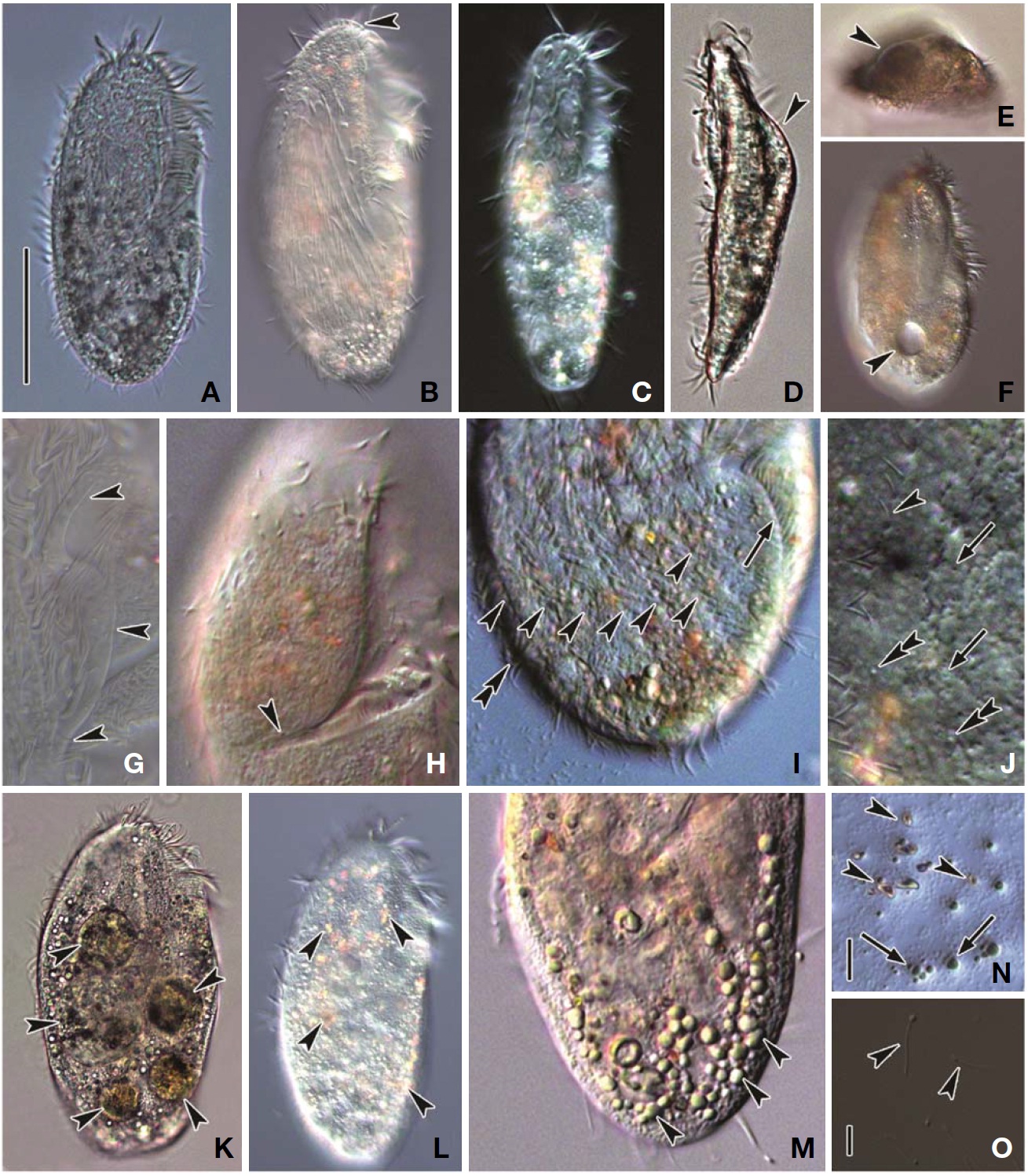
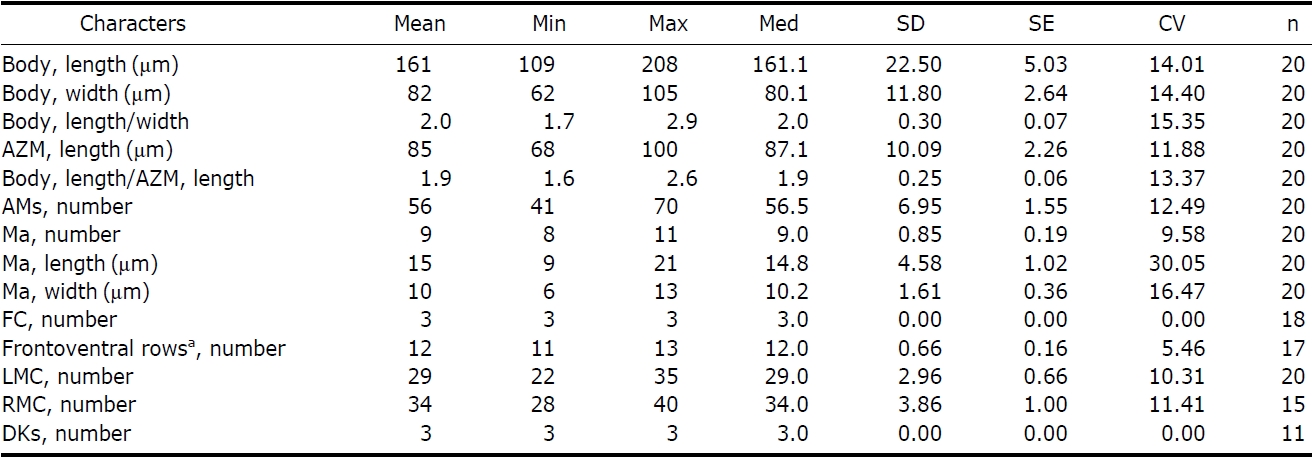
Description. General morphology and behavior: Body size 70-95×20-35 μm
Buccal field and oral infraciliature: Adoral zone of membranelles bipartited into proximal and distal portion by distinct gap (Figs. 1A, F, 3A), occupied 28-35% of body length and composed of 16-20 adoral membranelles (Table 1, Figs. 1F, 3A). Buccal area narrow and shallow, posterior half covered by projecting buccal lip (Fig. 1A). Paroral and endoral membranes parallel, endoral longer than paroral (Figs. 1F, 3E). Pharyngeal fibres distinct after protargol impregnation, about 20 μm(Figs. 1F, 3A).
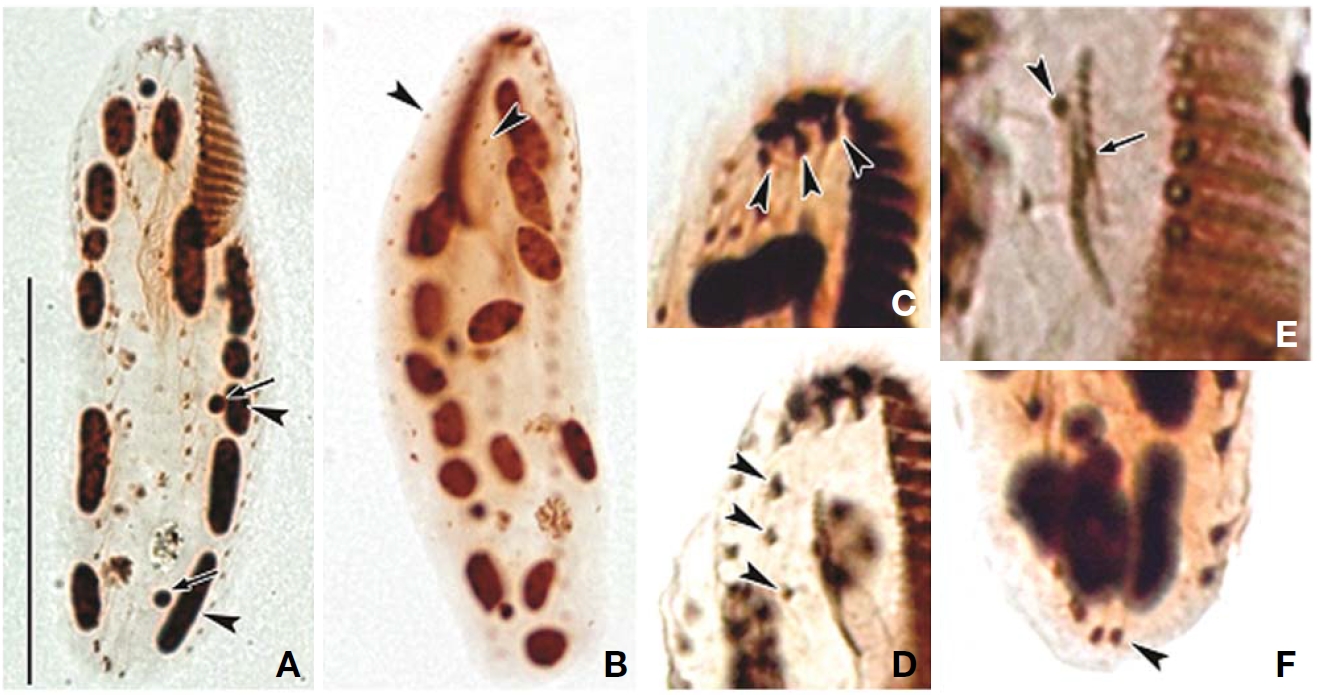
Somatic infraciliature: About 3-4, usually 3 frontal cirri (Figs. 1F, 3C). One buccal cirrus located at anterior end of endoral membrane (Figs. 1F, 3E). One row of cirri longitudinally arranged (frontal-row cirri) (Figs. 1F, 3D) below rightmost frontal cirrus, composed of 1-4, usually three cirri. Amphisiellid median cirral row commenced slightly behind rightmost frontal cirrus, comprising 14-21 cirri. Right marginal row with 21-30 cirri, extending posterior end (Fig. 3F), left marginal row with 10-19 cirri (Figs. 1F, 3A). Caudal cirri and transvers cirri absent. Dorsal bristles 2-4 μm long (Figs. 1G, 2H). Two to three dorsal kineties extending to posterior portion, rarely third short row present (Figs. 1G, 3B).
Cortical granules: Cortical granules spherical in shape, longitudinally arranged on both ventral and dorsal sides, slightly to obviously yellow in colour, about 0.5μm in diameter (Figs. 1B, C, E, 2I).
Nuclear apparatus: 11-20 globular to ellipsoidal macronuclear nodules, mostly arranged along cell margins, 3-10 μm in length. Two to five micronuclei, spherical, about 2 μm in diameter (Figs. 1G, 3A).
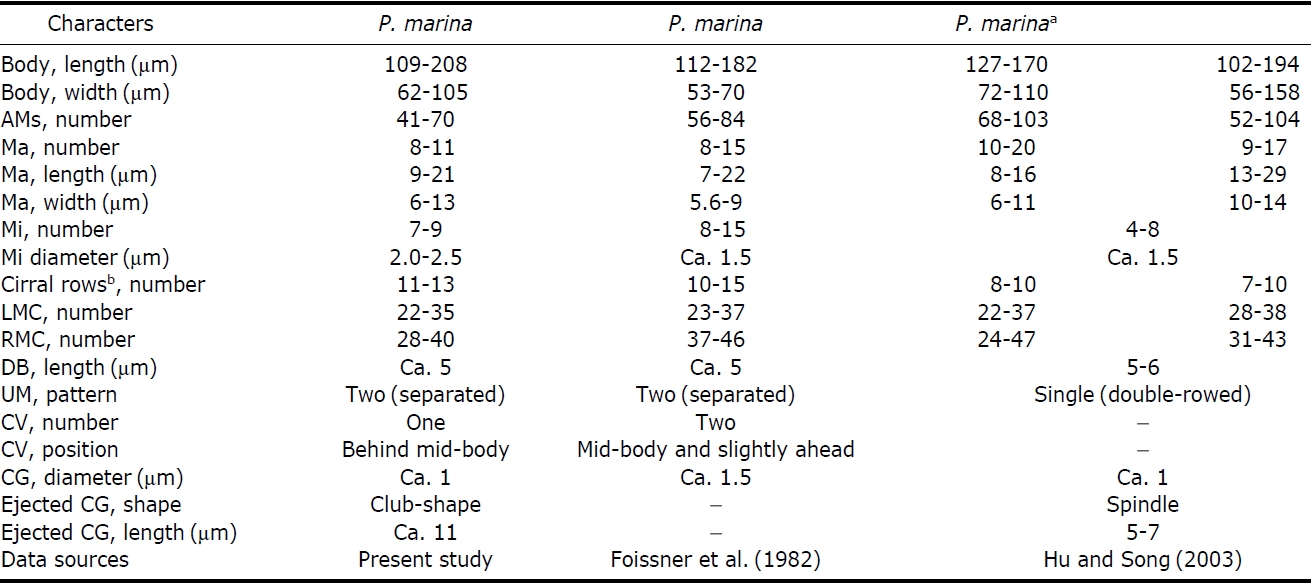
Distribution. Africa (Namibia), Asia (Korea [present study]), and North America (USA).
Remarks. The genus
The Korean population of
slightly different from the African population in the length of the right marginal row. In the Korean population, the right marginal row usually extends to the posterior end of cell, whereas in the African population, it does not. The shape of the cortical granules is slightly different in both Korean and the African populations (spherical vs. ellipsoidal). The Korean population of
1*Family Kahliellidae Tuffrau, 1979
2*Genus Pseudokahliella Berger, Foissner, and Adam, 1985
3*Pseudokahliella marina (Foissner, Adam and Foissner, 1982) Berger, Foissner and Adam, 1985 (Tables 3, 4, Figs. 4-6)
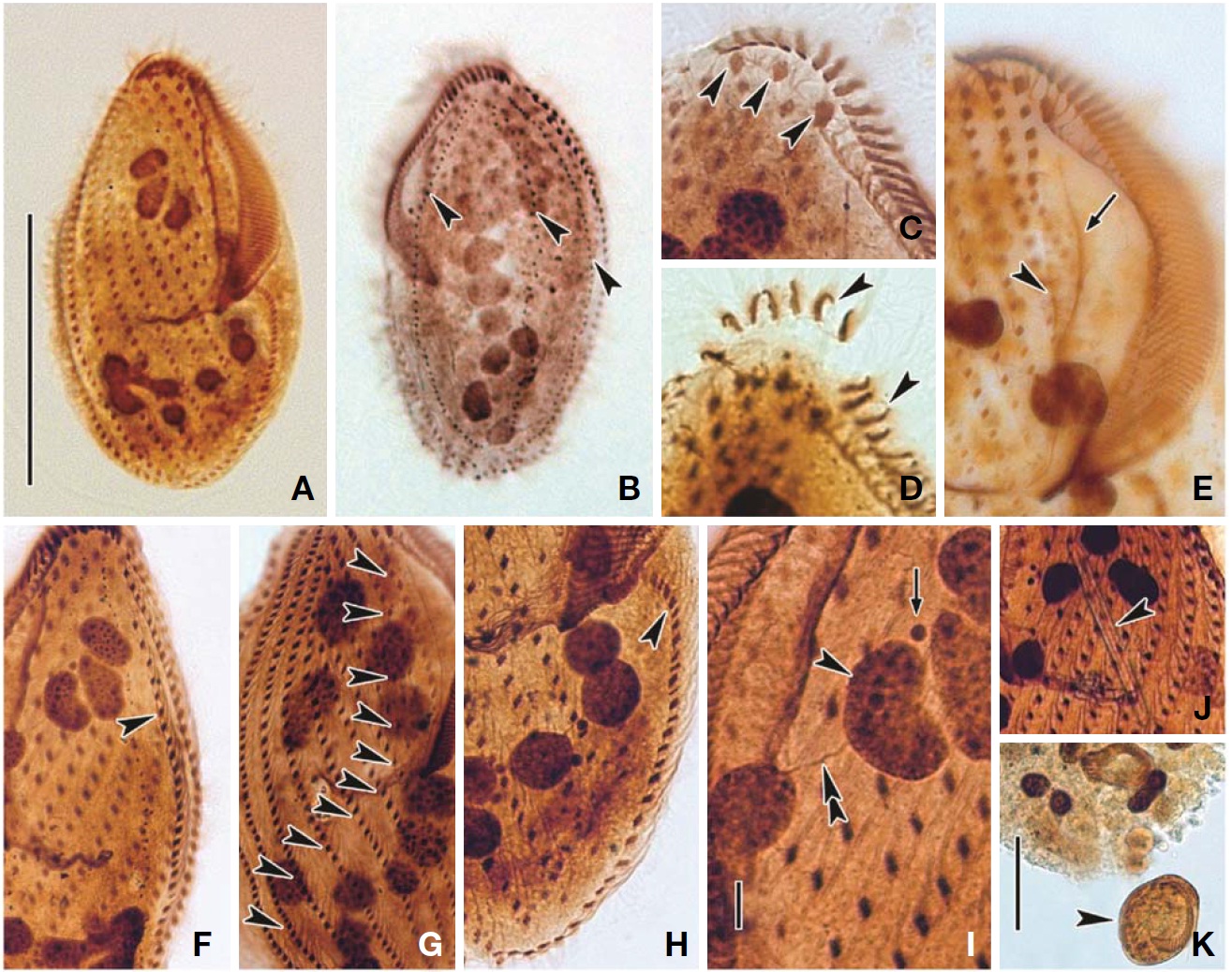
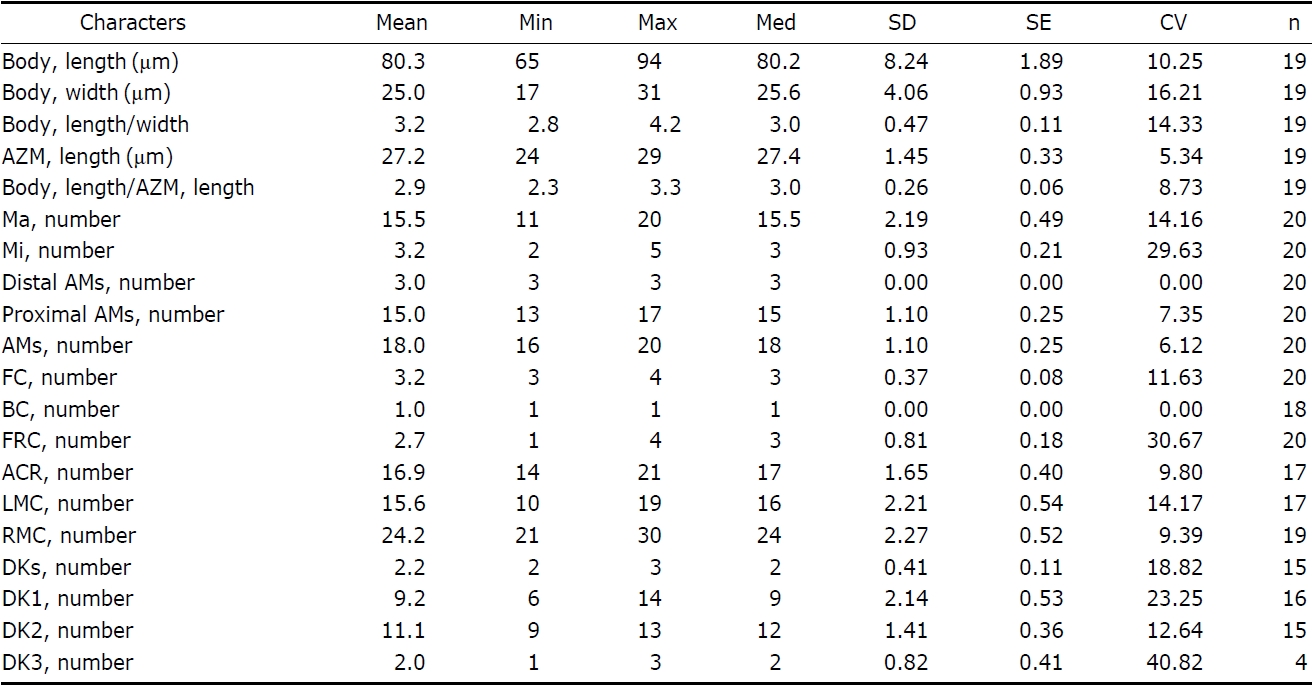
Description. General morphology and behavior: Body size 110-195×40-110 μm
an approximately 3 : 1 ratio; ventral side slightly concave; dorsal side distinctly convex (Figs. 4B, 5D, E). Prominent frontal scutum on anterior end of cell (Figs. 4A, C, 5B). Body very flexible but not contractile. Cytoplasm almost colourless at low magnification, including greenish shining globules and yellowish crystals (Fig. 5L-N). Single contractile vacuole spherical in shape, located below mid-body and slightly rightwards, size about 20 μm in diameter (Figs. 4A, 5F). Locomotion usually crawling on substrate and swimming spirally. Omnivorous feeding (Figs. 5K, 6J, K).
Buccal field and oral infraciliature: Adoral zone of membranelles occupied 50-60% of body length (Figs. 4F, 6A), continuous and semicircular, distal end extended to dorsal side of anterior end (Figs. 4F, G, 6B), composed of 41-70 adoral membranelles (Table 3, Figs. 4F, 6E), bases of widest membranelle about 13 μm in length. Buccal cavity narrow and rather deep (Figs. 4A, 5B, C). Buccal lip prominent (Figs. 4A, C, 5G). Undulating membranes long, straight to slightly curved (Figs. 4F, 6A). Paroral extended to near proximal end of adoral, about 60 μm long; endoral close to paroral, about 40 μm long (Figs. 4F, 6E). Pharyngeal fibres about 35 μm long and extended transversally (Figs. 4F, 5H, 6A).
Somatic infraciliature: Constantly three frontal cirri, near anterior end region, two slightly enlarged cirri connected to
[Table 1.] Morphometric data of Afroamphisiella multinucleata
Morphometric data of Afroamphisiella multinucleata
[Table 2.] Comparisons between different populations of Afroamphisiella multinucleata and A. abdita
Comparisons between different populations of Afroamphisiella multinucleata and A. abdita
the frontoventral cirral row but leftmost cirrus distinctly enlarged and displaced leftwards (Figs. 4F, 6A, C). Usually 11-13 frontoventral rows including left and right marginal row, arranged slightly obliquely backwards, each cirrus about 12 μm long (Figs. 4F, 6G). Left marginal row curved inwards in anterior portion, extending to posterior end, comprising 22-35 cirri (Figs. 4F, 6H). Right marginal row commencing from anterior one-third on dorsal side, comprising 28-40 cirri (Figs. 4G, 6F). Caudal and transverse cirri absent. Constantly three dorsal kineties with densely arranged cirri; bristle about
5 μm long (Figs. 4D, G, 5J, 6B).
Cortical granules: Cortical granules colourless about 1 μm in diameter, sparsely arranged on dorsal surface and longitudinally between dorsal kineties but not detectable on ventral side, (Figs. 4D, 5J). Completely extruded forms of cortical granules, about 10 μm long, club-like in shape, composed of spherical head and elongated stem (Figs. 4E, 5O).
Nuclear apparatus: Usually 8-11 macronuclear nodules, spherical to ellipsoid but rarely sausage-shaped, size 9-21×6-13 μm, connected to each other by thread-like structures, forming C-shape in dorsal view. 7-9 micronuclei, sphericalshaped, near the macronuclei, and 2.0-2.5 μm in diameter (Figs. 4G, 6I).
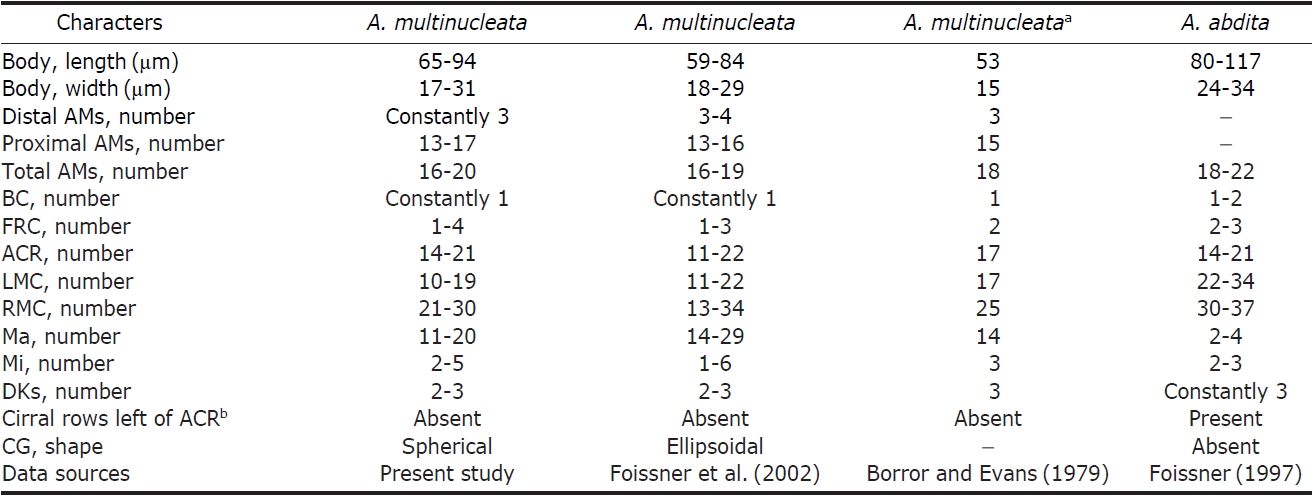
Distribution. Europe (France) and Asia (China, Korea [present study]).
Remarks. The genus
[Table 3.] Morphometric data of Pseudokahliella marina
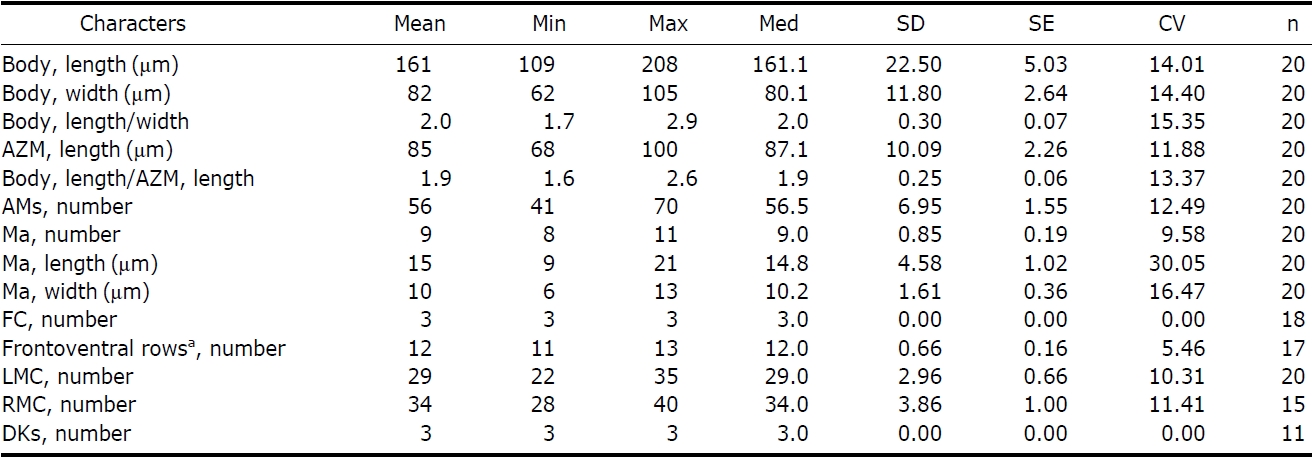
Morphometric data of Pseudokahliella marina
[Table 4.] Comparisons of different populations of Pseudokahliella marina
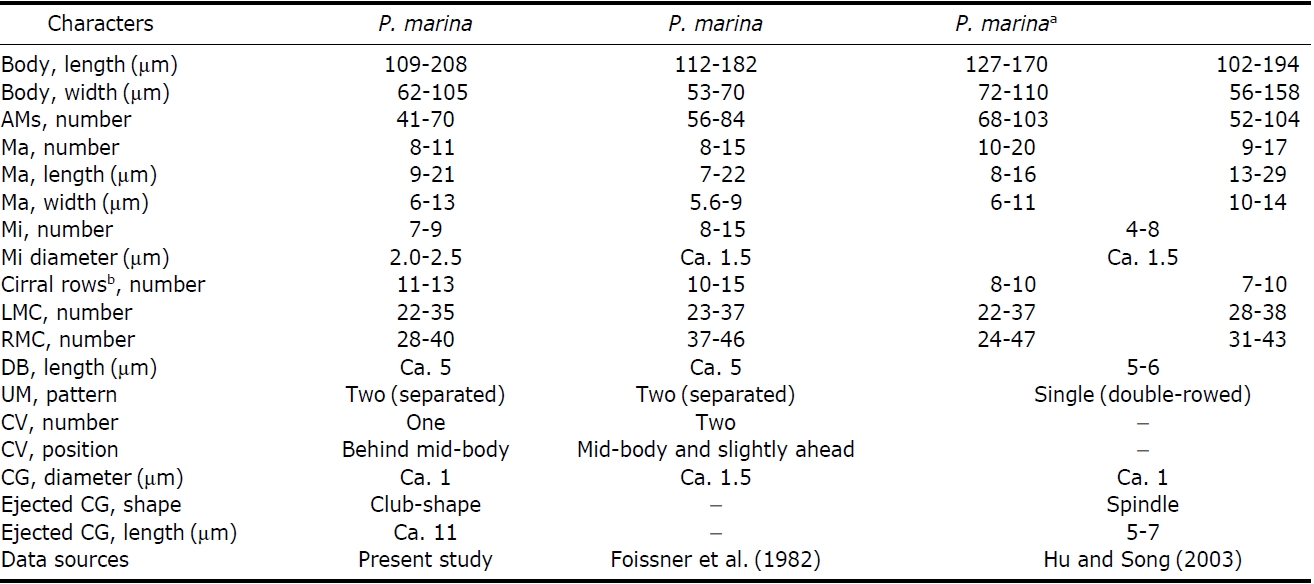
Comparisons of different populations of Pseudokahliella marina
μm); (4) diameter of micronucleus (2.0-2.5 μm vs. ca. 1.5 μm); (5) diameter of cortical granules (ca. 1 μm vs. ca. 1.5 μm); (6) number of contractile vacuoles (one vs. two); (7) position of contractile vacuoles (behind mid-body vs. midbody and slightly ahead) (Foissner et al., 1982; Berger, 2011). In addition, the following features are minor differences between the Korean and Chinese populations: (1) pattern of undulating membranes (two [separated] vs. single [doublerowed]); (2) number of cirral rows including both marginal rows (11-13 vs. 7-10); (3) shape and length of ejected cortical granules (club-like, ca. 11 μm vs. spindle, 5-7 μm) (Hu and Song, 2003; Berger, 2011) (Table 4).
Korean name: 1*아프리카양열하모충속, 2*다핵아프리카양열하모충
Korean name: 1*많은측극모열과, 2*많은우측극모열충속, 3*많은우측극모열충