People are willing to spend for their health. World-wide, healthcare costs have been constantly rising for decades. In U.S., expenditure on healthcare surpassed $2.3 trillion in 2008, more than three times of the $714 billion in 1990 [1]. Per resident the cost for healthcare is about $7,681. This accounts for 16.2% of the nation’s gross domestic product (GDP). In China, the health expen-diture accounts to about 4.7% of the GDP [2]. It rises at an annual rate of 15%. This is much faster than China's economy growth and the other developing countries.
As China’s population ages, there is an increase in the need for public medical services for outpatient monitor-ing, chronic disease management and elderly care. It is suggested by the National Bureau of Statistics of China that the proportion of the population aged 65 and over is projected to rise remarkably from 7.87% in 2008 to 11.92% in 2020 [3]. In the middle of the 21 century, the proportion will rise to more than 25%. Heart disease, the leading cause of death in many rich counties, kills one person in every 15 seconds in China alone. Among these deaths, about 70% are due to the lack of appropriate long-term care and monitoring. Thereby, it fails to provide the right treatment in the very first minutes of a heart attack.
Traditional medical services can be featured as hospital-centric. It provides services in a reactive manner. Patients go to the hospital when they experience certain symptoms. This service model exhibits many critical limitations for heart diseases as the circulatory system is a complex sys-tem. It has a self-recovery capability and it may recover the abnormity to some degree. This results in a disappeared symptom when the patients arrive to the hospital. Notice that this recovery procedure is common. When the recov-ery fails, it will result in a heart attack. It often experi-ences a long time from the onset of a circulatory disease to a final heart attack. In this sense, a traditional medical service model can hardly provide satisfactory treatment experiences for cardio system patients. Due to this need, we have developed a 24-hour real-time heart care and monitoring system called as CardioSentinal and we will report on some of the recent progresses on the Cardi-oSentinal system in this article.
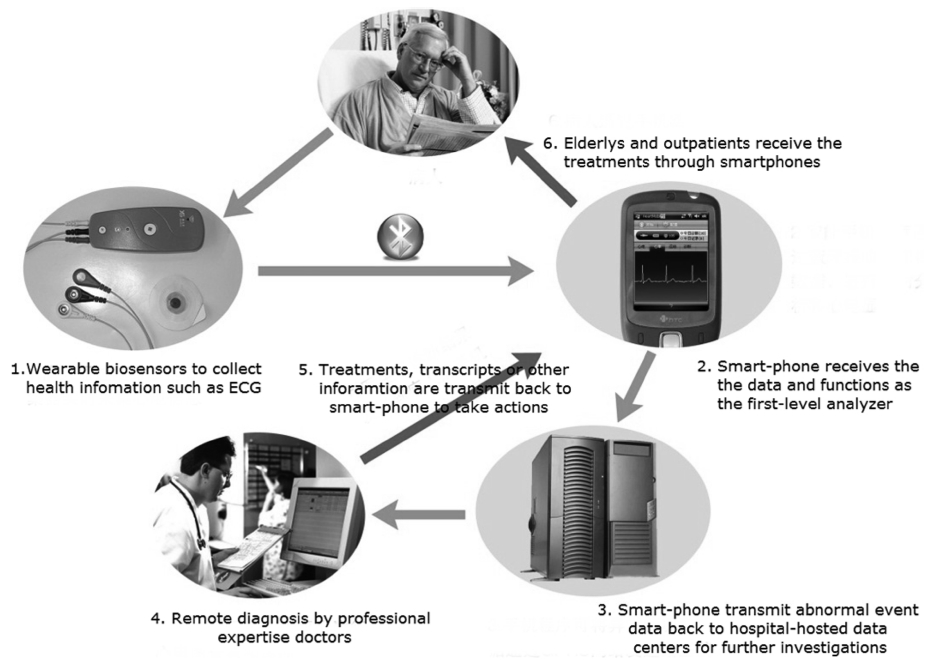
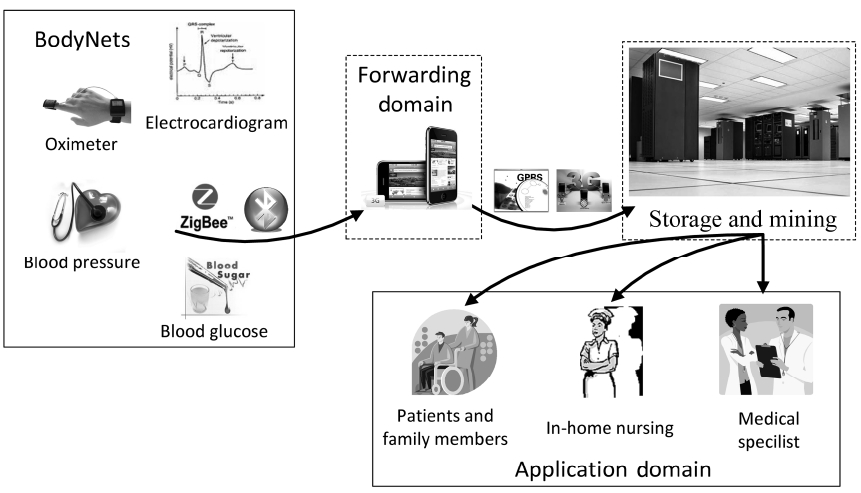
CardioSentinal is an interdisciplinary system that inte-grates recent advancements from various fields. Fig. 1 depicts the general architecture of the CardioSentinal sys-tem. In CardioSentinal, portable body sensors are we ara-ble to patients with sustainable battery power. Sensors vary according to the application demands such as the electrocardiogram (ECG), oximeter, blood pressure and blood glucose. Other non-bio-sensors are also greatly helpful for daily monitoring purposes such as the temper-ature, humidity and motion (3D accelerometers). They are interconnected through short-range wireless commu-nication channels (e.g., Bluetooth) forming a body are a network (BodyNet). They deliver the collected informa-tion to a nearby personal digital device such as a smart-phone. These smartphones serve as a data forwarder and a first-level analyzer. If some suspected information has been identified then, the smartphone will report to a data-
center through a 2.5G/3G cellular network or WiFi and notify medical doctors for an expert check. As the smart-phone is attached to patients, doctors can easily access the patients and further actions can be taken immediately. All these processes can be carried out in a non-intrusive manner. So, no interaction from the patients is required.
According to the typical application requirements, there are various design goals of CardioSentinal. The first one is the system response time. When an abnormal event occurs, corresponding actions should be taken immedi-ately. Otherwise, damage may occur to patients or it may even lead to casualty. The second metric is the accuracy. It is measured in two ways. One is the false positive (FP) in which the system gives an alert when the user is healthy. The other is the false negative (FN) which hap-pens when the user is in danger but the system fails to capture the event. Usually there is a tradeoff between the FP and FN rates. FP is acceptable and a low rate is desired in order to reduce un-necessary bothering to users. But, FN is unacceptable as disastrous consequences may take place. The third metric is the energy consumption and system lifetime. Batteries can be replaced and recharged but frequent recharging will dramatically com-promise on the system attractiveness. Besides these user-centric metrics, there are also other system-centric met-rics such as the system robustness, communication reli-ability, security and privacy protection.
During the design and implementation of CardioSen-tinal, we faced several technical and practical challenges. The first challenge was related to the body sensors. It had to provide reliable sensing readings in daily harsh envi-ronments. For example, the environmental electrostatics will introduce great errors and cause deviated readings that can hardly reflect real measurements. The second challenge was the smartphone. We needed to implement complex data-mining algorithms on top of simple and computational-limited hand-held platforms. The third chal-lenge was the back-end data center. It was facing a great challenge of dealing with a large scale of online stream-ing measurement data in real time.
The rest of this paper is organized as follows. In Section II, more details on the application background are given. Some existing efforts including those from the research academia as well as the industry will be given. In Section III, we elaborate the details of the CardioSentinal design and we focus more on the individual system components and the challenges we are facing. In Section IV, we pro-vide the field and clinic test results for CardioSentinal and draw a conclusion in the last section.
II. BACKGROUND AND RELATED WORK
Healthcare is one of the major applications in wireless sensor networks. Stankovic directs the laboratory for real-time and embedding computing in the Department of Computer Science, University of Virginia. His group develops the Alarm-net system that integrates the various sensing devices that are available inside the living space to perform health-missions [4]. Fig. 2 graphically depicts the Alarm-net cores. It adopts MICAz [5], a wireless sen-sor node as the fundamental platform. It also provides pervasive and adaptive healthcare monitoring by using environmental and motion sensors. It extends healthcare from the traditional clinic and hospital setting to an assisted-living and retirement homes. Alarm-net mainly focuses on the context of the target users (typically the elderly and outpatients) with an intensive interest in motion, light intensity, humidity, object (user) locations and mobility. It offers neither physiological sensing nor instant disease diagnosis. The system is still bulky and it is inconvenient for practical application. Another impor-tant work is carried out by European researchers under the 7th framework program [6] called Opportunity [7]. It develops algorithms and architectures for activity and context recognition. The objective is to develop mobile systems to recognize human activity and user context with dynamic sensor configurations.
Due to the importance and the profound effects of
BodyNets to the healthcare, there is an increase in the interest on BodyNet research. The researchers in the Imperial College London study the context-aware and environment-aware BodyNets [8]. Yuce et al. [9] in UC Berkeley studies the wearable BodyNets and it pays more attention on the system scalability and resource optimiza-tion. Chan et al. [10] in CUHK studies on the multi-band communication BodyNets, mobile BodyNets and energy-aware MAC for BodyNets. Yun et al. [11] studies the energy-efficient communications for BodyNets. NUS group studies the architecture of BodyNets and proposes their own architecture [12]. More research works have also been conducted on the BodyNet adaptability, self-organi-zation, middle-ware, signal processing, system reliability and healthcare and monitoring [12-15]. There are also some promising BodyNet research projects such as Code-Blue [16], MobiHealth [17], MIThril [18] and MyHeart [13]. These works share the similar philosophy of Cardi-oSentinal but they are focused on different aspects of the remote healthcare. They provide valuable information dur-ing our system design and implementation.
Many medical sensors for physiology monitoring are commercially available and off-the-shelf products can be easily accessed. For instance, Omron sells a heart rate monitor HR-100C [19] that can accurately measure the heart rate. It is portable and it is suitable for measurement during sports and physical training. However, it provides no information about whether the user is healthy or not. Alive Technology Pty Ltd [20] located in Australia pro-duces a product that shares a similar concept of Cardi-oSentinal. It has an attachable sensor to acquire, transmit and record the ECG and acceleration signals of the patient. It uses Bluetooth for short-range communications. This product is just a data gathering device. It provides no intelligence on patient diagnosis and context analysis. It is limited in analysis of potential dangers in patients. Thus, its usage is limited. It has no energy-saving or reliable communication design. Thus, it may fail in practical harsh environments.
In this section, we present in details the system design of CardioSentinal. We first give the general system archi-tecture and we focus more on the design challenges in each of the system component. We then, describe the design and implementation of each component in detail.
The system is mainly composed of three subsystems, i.e., the BodyNets, the smartphone gateway nodes and the remote data centers (Fig. 3). The BodyNets are used to collect the physiology data by wearable biomedical sen-sors and they are carried by users, typically the elderly
and outpatients. According to different application require-ments, these sensors can be attached to the human body or planted in the human body. The biomedical sensors have two basic modules namely, the sensing module and the communication module. The communication module will communicate with the nearby gateway nodes in order to upload the sensory data.
Gateway nodes are typically smartphones, tablets or even desktop PCs which have the shot-range communica-tion capability. They aggregate the data and conduct a first level data analysis function. They render the data on the display in real-time and they function as the first-level data analyzer. These gateway nodes also subscribe to the Internet and this allows them to deliver the data to back-end data centers upon abnormal events. Moreover, they are observed by the analyzer. The data centers are usually hosted and maintained by the hospital and other medical organizations. Professional doctor expertise can check the online data as well as the historical data. When neces-sary, they can even control the remote biomedical sensors in order to collect more customized information (in cur-rent generation, this function is not yet implemented). Doctors will suggest treatment plans for users and down-load it to the gateway nodes. Users can then access these suggestions and interact with the doctors.
In the current version of the system, we develop the system based on the HTC S1 model. It runs the standard windows mobile phone 6.1 operation system. The Blue-tooth version is 1.1 profile [21]. We designed all the nec-essary communication protocols. Currently, our system can support up to three biomedical sensor modules for each smartphone but in practice users are likely to pair the sen-sors and phones one-by-one for usage convenience.
In the next subsections, we will present the challenges and designs in the three components respectively.
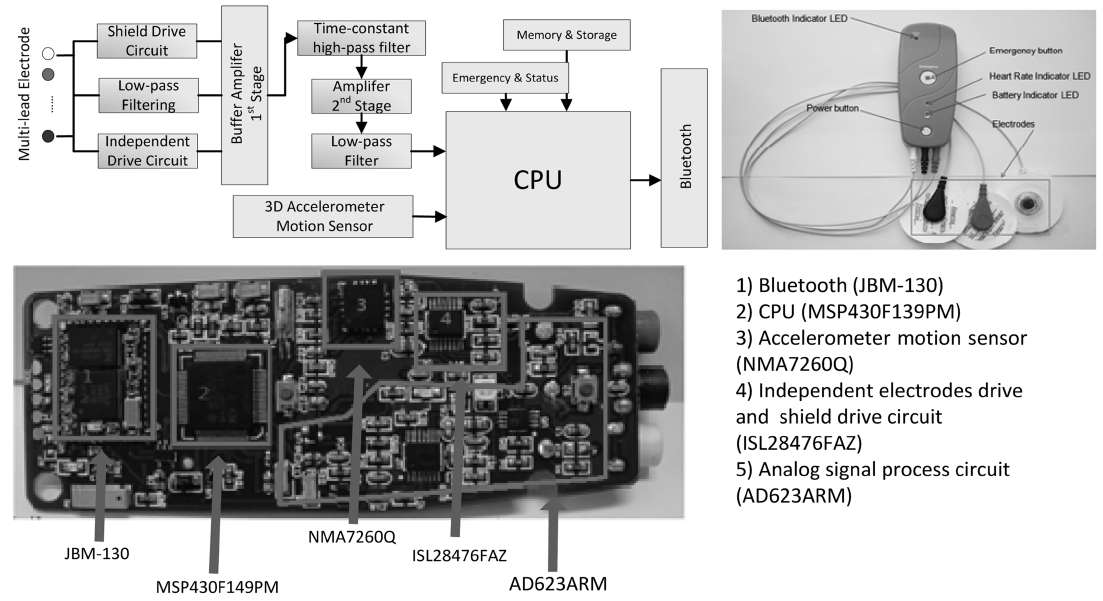
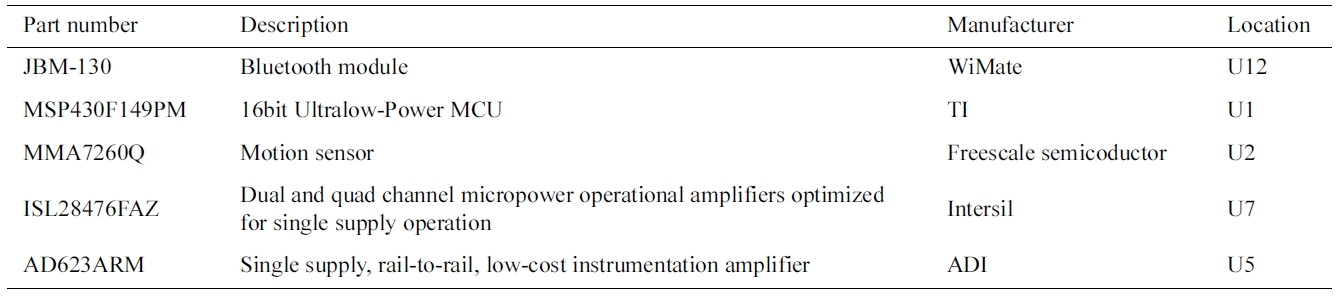
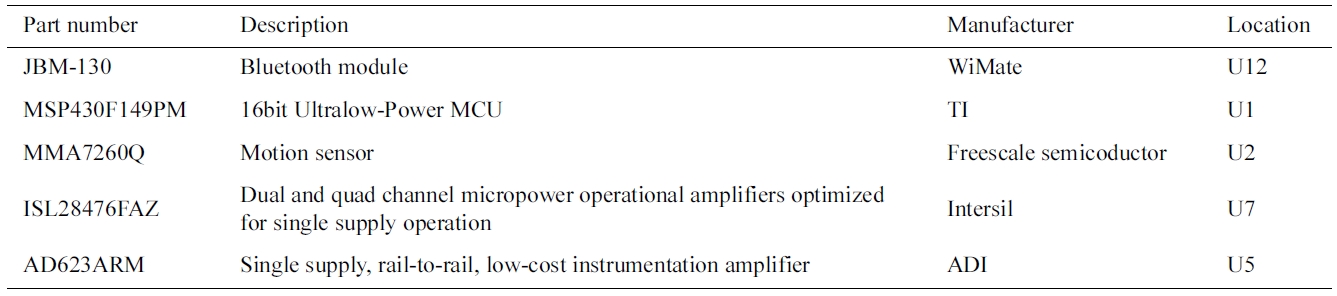
In the current generation of CardioSentinal, the bio-medical sensors integrate ECG sensors, environmental sensors and motion sensors (accelerometers) and pack them to a portable package. A diagram of the biomedical sensor, the product and the key printed circuit board (PCB) are shown in Fig. 4. We listed all the parts that were used in this design in the Table 1 including the part number, the functions, the manufactures and the loca-tions. In what follows, we will describe in detail the implemented functions in one package. An example of the ECG signal measurements and the user’s present motions are depicted in Fig. 5.
1) Low-power ECG Signal Measurements
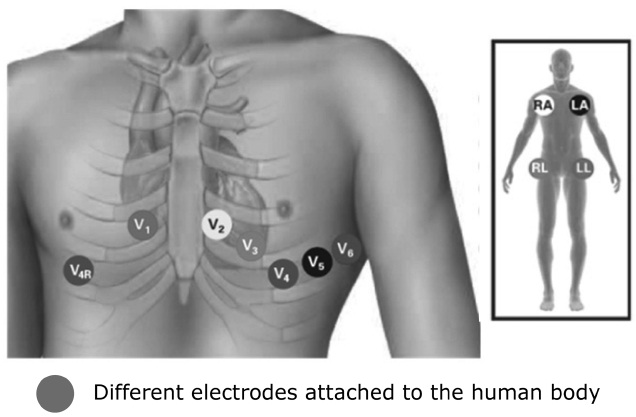
Normally, the activities of the heart begin in the sinoatrial node. The sinoatrial node originates the electri-cal impulses that travel through the intrinsic conducting system to the heart muscle. These impulses stimulate the myocardial muscle fibers to contract and induce systole. So, we can measure the electrical currents by placing electrodes at certain points on the skin. Between the patients (users) and the ECG devices are patient wires. Wires are many and they range from 3 to 10. These wires lead to different lead ECG systems. A lead is a view of the electrical activity of the heart to form a particular angle across the human body. It is obtained by using the
Part list
different combinations of the wires. Individual wires pro-vide no physical meaning while each lead traces the heart’s electrical activity between the two points. For a 12-lead ECG (e.g., Fig. 6), 10 wires are needed that are attached to the different positions around the limbs and chest. In general, high lead ECG systems provide richer
information for heart activity but introduce more incon-venience and power consumptions. More details of the system installation and operation of the electrodes can be found in the system’s user manual.
2) Motion Detection and Identification
Different from the Holter ECG systems that are used in hospitals, CardioSentinal targets at 24-hour heart moni-toring purposes. Besides the typical heart electrical activ-ities that are obtained by the ECG machine, we are more interested in the context information of the users when the ECG measurements are collected. More specifically, we detect and identify the motions of the users during the run time. This can greatly benefit the diagnosis based on the ECG information. For example, during exercises, say walking on a treadmill or pedaling a stationary bicycle, we will observe a lot of artifacts such as the baseline drift, which are all typically normal. But, these artifacts will imply very dangerous activities when it is sleep time.
To accurately separate such circumstances and provide better user experiences, we integrate 3-dimensional accel-erometers in to the portable ECG machines. As the ECG machines will be continuously carried by the users, these accelerometers can measure the accelerations of the users and help us imply the motions of the users. 3-D acceler-ometers can detect both the magnitude and directions of the accelerations and the output as a vector quantity. It helps us to identify the orientation, vibration and shock motions of the users. Notice that the ECG machine only collects the raw data and transmits it back to the smart-phone data analyzer. The data is analyzed and the infor-mation is obtained by the smartphone in order to improve the ECG analysis accuracy. Currently, the motion data is presented for only the reference of a doctor. Motion arti-facts by online ECG data recognition algorithms are still open challenges and hot research topics and they are left for future work.
Considering the hash wireless environments for short-range wireless communications, we install a built-in secure digital (SD) storage in the ECG to store the un-uploaded data. The current off-the-shelf SD storage is quite large and it concerns the typical 200 MB data for 3-lead ECG system per day, a cost-effective SD storage can store up to months of raw data.
3) External Interference Filtering
A major issue in the implementation of the ECG sys-tem is the external electrical interference. Electrodes measure the electrostatic level on the skin that is induced by the heart electrical activities. However, radio interfer-ence such as the electrostatics can also be induced by the external factors. One is the frictions of clothes and this will also generate a great deal of electrostatics. This can partially be dealt with by a careful electrode design that provides certain degrees of protection. We also notice that the external electrostatics often show high variations. This is mainly due to the friction electrostatics that are generated and affected by too many environmental fac-tors. Such high variations will exhibit high frequency property at the measurement frequency domain. Another main external interference is by the power grid electric-ity. The power grid frequency is standardized world-wide as 50 Hz or 60 Hz. Their frequency is coincident with the human pulse frequency and it ranges from 40 Hz to 120 Hz.
To deal with these issues, we take the following approaches. We design a low-pass filter circuit for all stage amplifiers to attenuate non-cardiac electro-biologi-cal signals. A time-constant and high-pass filter circuit is implemented to eliminate random polarization voltage. Thereby, it stabilizes the baseline of the input signal. In the first stage amplifier, we use instrumented operational amplifier in order to increase the input impedance of the circuit and its common-mode rejection ratio. As such we enhance the circuit anti-interference capability. We also design independent electrode driving circuits for each of the electrode wires in order to improve the circuit com-mon-mode rejection ratio and we restrain the frequency interference. Thereby, we stabilize the baseline. For the whole ECG package, we encapsulate it into a shielded driving circuit in order to restrain frequency interference and electromagnetic interference outside. With all these means, the impacts of the external interference on ECG measurement can be dramatically negated.
4) Cooperative Short-Range Communication
With the development of the portable remote health-care systems, a single user may carry multiple indepen-dent biomedical sensors. As illustrated in Fig. 1, four different kinds of sensors cooperatively help in the col-lection of physiology information of a human body. A naive communication scheme in such multiple biomedi-cal sensor scenarios is to let them transmit independently is better and the sensors are directly connected to the smartphone. However, practically this can be quite ineffi-cient as biomedical sensors are deployed in a wireless harsh environment. The link qualities between the bio-medical sensors and the gateway smartphones dramati-cally vary due to shadowing and multi-path effects of the human body. As short-range communication protocols such as Zigbee and Bluetooth often have very limited bandwidth, the poor quality wireless links have to trans-mit multiple times and adopt a relatively low data rate. This will waste the valuable transmission airtime.
A smart transmission scheme that is suitable for the harsh BodyNets is cooperative communication. We lever-age spatial diversity and allow different biomedical sen-sors to share their antennas. As such we can construct a virtual multiple-input and multiple-output (MIMO) sys-tem that overcomes the limitation of a single antenna. For a simple illustrative example, suppose there are two bio-medical sensors A and B and one gateway node C. Links AC and BA are quite good while BC is poor. Rather than directed communications from B to C, allowing A to relay B’s data can dramatically reduce the transmission time and energy consumption.
Cooperative communications can be done in decode-and-forward, amplify-and-forward or coded cooperative manner. The first decode-and-forward manner is more suitable for high link quality scenarios as incorrect decode will be propagated and it will result in more errors. On the contrary, the amplify-and-forward suits more for the poor link quality scenario but the noise will also be amplified together with the signals.
Among the three, coded cooperative communication provides the best flexibility and it usually outperforms the other two but it is the most complex communication system as well. It often requires the transmitter (B in the example) and there lay node (A in the example) to share the link quality information. This is quite a challenge and it may introduce a lot of control overhead.
The smartphone mainly has three functions namely data storage, first-level and online data analysis, and data forwarding (to back-end data centers when necessary). One of the major challenges is wireless communication. The connections between a smartphone and back-end servers are through general packet radio service (GPRS) or 3G and they are quite reliable. However, the connec-tions between the biomedical sensors and the smartphone are through the unreliable Bluetooth channels. We need to build reliable data links upon such unreliable, easily interrupted Bluetooth channels. The second challenge is the online heart disease analysis. This is a first-level anal-ysis as it is neither practical nor economical to transmit all raw data to the back-end data servers for diagnosis (recall that connections between the smartphones and the Internet are expensive). Concerning the limited computa-tional capability and the storage space of the smartphone, we designed a tailed data analysis algorithm to fit the smartphone platform. In what follows, we will describe the design of the two parts. Recall that the energy con-sumption is always a concern during design.
1) Wireless Data Transmission
Bluetooth protocol is a mature technology which has been widely used in smartphones. Naturally Bluetooth channels are built upon unreliable physical layer proto-cols but data reliability is extremely important for our applications. Therefore, we design and implement a tailed, lightweight transmission control protocol (TCP)-like pro-tocol for reliable wireless data transmissions. The basic idea is that data are packaged to fixed-size data streams. Packet IDs are assigned to the data stream so that lost packets can be retransmitted.
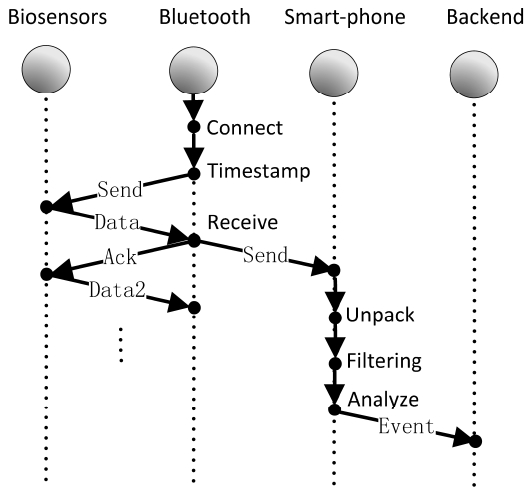
The detailed communication flowchart is depicted in Fig. 7. When connected to the smartphone, the Bluetooth part of the biomedical sensors will send a timestamp to
the data collector unit in order to obtain the raw data and acknowledge the sensing unit. This happens within the biomedical sensor unit. The abovementioned procedure will continually conduct until a certain command asks or lets are better than requests the biomedical sensors to stop sensing (say power-off). Bluetooth will send the message to the smartphone which unpacks, filters and analyzes the data. When abnormal symptoms are observed, the smart-phone will define an event based on the data and send the event back to the data centers. When the event is affirma-tive by the data center part, the data center will ask the smartphone to compress and upload all the stored data for further analysis.
2)First-Level Data Analysis
For further data analysis, the smartphone will conduct a first-level data analysis to preprocess the data. With the cardiology domain knowledge, the ECG waveforms con-tain five typical deflections. They are arbitrarily named as P to T waves. The Q, R, and S waves occur in rapid succession and they all do not appear in all leads. More-over, they reflect a single event. These three are usually considered together. AQ wave is a downward deflection after the P wave. The R wave follows as an upward deflection and the S wave is a downward deflection after the R wave. The T wave follows the S wave. In some cases an additional U wave follows the T wave.
The ECG waveform contains all these five deflections if any. It also contains external interference such as the industry power line interference, electromyography (EMG) from muscles, motion artifact between the electrode and skin interfaces and other interferences from electro-sur-gery equipment. The first-level data analysis mainly focuses on the removal of these interferences and it reconstructs a clean ECG data.
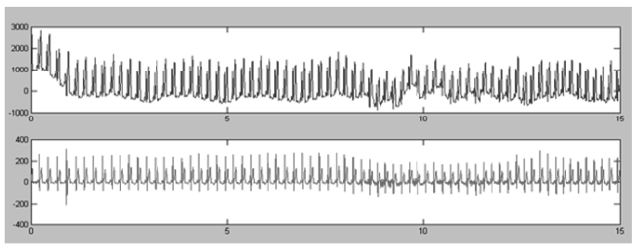
An aggregated effect of these external interferences is
the so-called baseline drift. An example of the baseline drift is depicted in Fig. 8. Due to the limited computa-tional capability of the smartphone, we adopt a simple median filter to remove the baseline drift. The idea is to use the median to estimate and extract the baseline and subtract the original signal from the obtained baseline. As such the baseline drift can be calibrated. Though the method is quite simple, it can effectively remove most of the drift and an example of the result is depicted in Fig. 8.
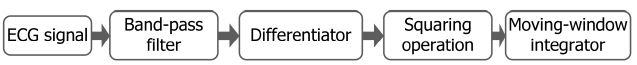
Another major task of the first-level data analysis is the R-wave detection. By Pan-Tompkins algorithm [22], we can detect the R-wave by using the equations that are given below. The ECG measurements will be passed by a low-pass filter and a high-pass filter which result in a band-pass filter. The filtered data will then pass a differ-ential function and a window function to compute the mean square. After an integration function, a threshold-based function will output the final R-waves. These pro-cesses are depicted in Fig. 9. The functions that are men-tioned above are listed below. Note that
Low-pass filter (LPF) function:
High-pass filter (HPF) function: yi = yi-1 + xi - xi-32
Differentiation:
Moving-window integrator:
With data preprocessed, certain simple symptoms of the ECG deflections can be identified. When more analy-sis of the data is required, the smartphone will transmit the data back to a data center for more profound analysis. At the data center end, we adopt machine learning classi-fication algorithms in order to identify more ECG deflec-tion patterns. The machine learning classification algorithms will help in the generation of expressions that can auto-matically assign each instance to a particular class. Numer-ous machine learning methods can be applied for supporting decision making in medical domains such as probabilistic, statistical and many others. Of these meth-ods, Naive Bayes, decision trees, logistic regression and neural networks are among the most relevant to our work.
An artificial neural network is a mathematical model for information processing and it is based on a connec-tionist approach. Back propagation (BP) networks and radial basis function (RBF) networks are both well-known variants. Both can learn arbitrary mappings or classifica-tions. In an RBF network, the activation of a hidden unit is determined by the distance between the input vector and the center vector of the hidden unit. The weights that connect the hidden layer and output layer can be deter-mined by a linear least square (LLS) method. Therefore, RBF networks can gives lightly higher classification accuracy for cardiac diagnosis compared to BP networks. We implement a RBF network with the k-means cluster-ing algorithm in order to provide the basic functions. For more complex practical situations where the goal is to estimate the probability of arrhythmia, mixture models may be more appropriate due to the iterative nature of fit-ting such density functions.
In this section, we present some field study results to demonstrate the effectiveness of CardioSentinal system. We applied the system in two major hospitals in Sichuan province, China, namely the Sichuan Provincial People's Hospital [23] and the West China Hospital [24]. With col-laborations with these two hospitals, we hired 46 patients and 68 volunteers. The volunteers from each hospital will form one group of data to test our system. Each test is carried out for a single day, i.e., 24 hours. For compari-son, patients also carry an off-line professional ECG device at the same time to collect more accurate data. The model number is ECG-6951E, manufactured by Shanghai Medi-cal Optical Electronic Instrument Co., Ltd [25]. Medical doctors will check the ECG-6951 data manually and gen-erate professional reports for the results. Though these results may also contain errors, they are the best that we were able to obtain. Thus, it will be used as the ground true for our CardioSentinal System. In the next, we will present some of the representative results.
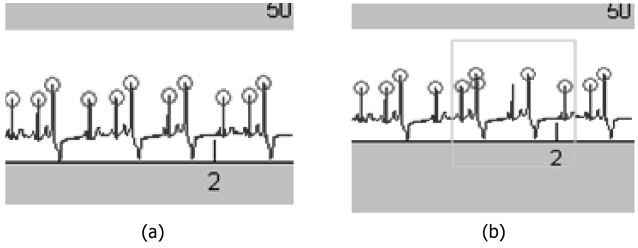
Fig. 10 shows the R-wave detection by a traditional algorithm and the results with our calibrations. In gen-
eral, when ECG signals encounter a premature beat, the R location to be tested in the next wave will be brought for-ward. It will cause a misjudgment (Fig. 10a, green area). This is a false detection. We found out that such false detections can be avoided when the premature beat and compensatory gap appear in pairs. We also found out that an appropriate threshold of the R-wave detection can also help in the reduction of the false rate. By using traditional Pan-Tompkin’s algorithm [22], the threshold selection is dynamic. The value is obtained from the average of 700 points. Certain degree of redundancy on the threshold (10% in our work) can effectively reduce the error.
In our field study data, there are around 40 to 50 R-wave false detections in the all-day ECG data. In MIT-BIH database (S20022.dat), there were 54 missing data and 211 redundant judgments. This reduces to 7 and 113 by using our algorithm. The correct rate rises to up to 99.76%. Notice that the false detections cannot be com-pletely eliminated. It naturally has a tradeoff between miss detections that are unacceptable.
>
B. Miss Detection and False Alarm
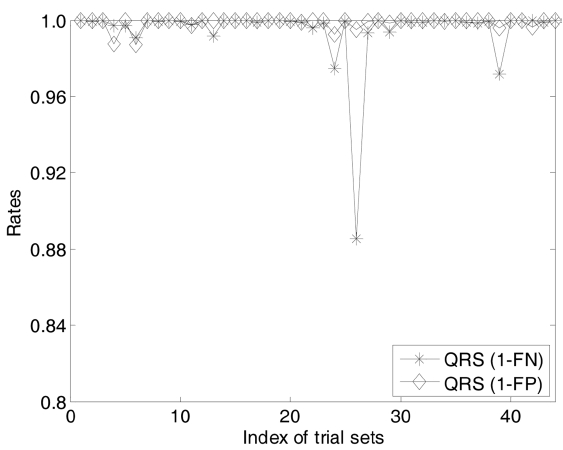
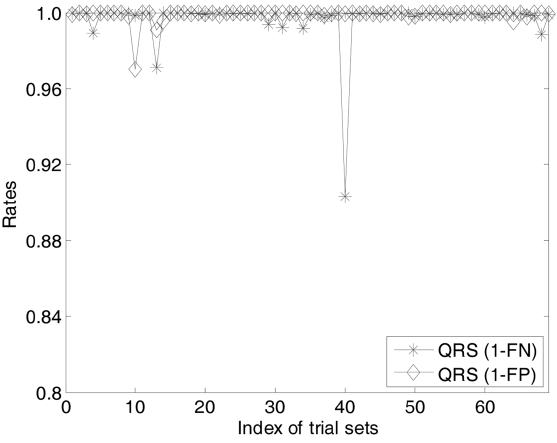
In this section, we investigate the diagnosis perfor-mance of CardioSentinal system. We mainly focus on two evaluation metrics, i.e., the miss detection measured by the FN rate and the accuracy measured as the FP rate. Due to the space limitation, we only report the evalua-tions on the QRS complex analysis and V-wave analysis with the two sets of data. Fig. 11 shows the sensitivity of the first data set measured by the 1-FN and the accuracy measured by 1-FP. In this set of data, there are 46 groups of raw data. For sensitivity, it is critical for the applicabil-ity of CardioSentinal. Moreover, in all the measurements there are only two cases with 97% sensitivity whereas all the other measurements are more than 99%. As there is a natural tradeoff between the FP and FN, the accuracy is much relatively lower than sensitivity. There is only one case with 88% accuracy and two cases of 96.8%. Others are all higher than 98%. Similar results are observed in the second data set while this time, we have one case of 96.5% sensitivity and the accuracy is slightly better than that in the first case (Fig. 12).
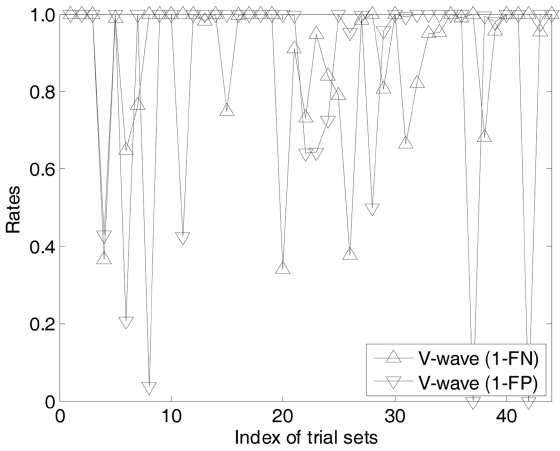
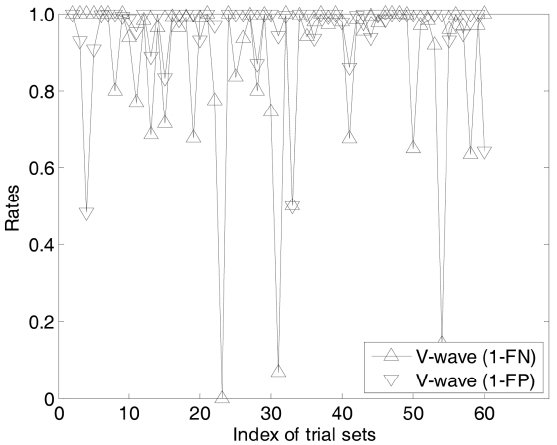
Figs. 13 and 14 show the evaluations of the V-wave detection on the same data sets. The V-wave detections
are much more difficult than QRS analysis. In most of the cases the sensitivity is more than 40%. In most of the cases, the sensitivity is more than 40%, while we also observe one case with the sensing accuracy as low as 0 and two cases with the accuracy as only 20%. Others are more than 60%. Notice that in the cardiovasology, auto-matic V-wave detections are still hot research topic. In clinical studies, the automatic detection algorithms are mainly meant for a doctor’s reference only and the final decisions can only be left to the doctors.
In this paper, we described our recent efforts on a remote healthcare system. We introduced the design and implementation of a CardioSentinal system. CardioSen-tinal is an on-demand 24-hour heart care and monitoring requirement for the elderly and outpatients. Users carry portable biomedical sensors that integrate several sensors such as ECG machine, motion sensors, humidity and temperature in order to obtain a full context of the heart activity. Bluetooth modules help the biomedical sensors to transmit the physiology data to nearby smartphones. Then, they analyze the data and deliver the abnormal events to the back-end data centers.
CardioSentinal is designed for daily family usage and it can dramatically improve the professional healthcare coverage. Clinic trials show that the current sensitivity and accuracy are as high as 99% with few cases being less than 96%. From the professional perspective, higher sensitivity and accuracy is expected and this will be our future research direction.
The current system is designed based on windows mobile system at the smartphone. Nowadays, the android systems and iOS systems are accounting more market shares. Our next major work is to immigrate the system to more open android platforms. In the current design, we mainly focused on the system robustness, communication reliability and the accuracy. Yet there is no special care on the energy consumption or security issues and they will be addressed in the future generations.
![Alarm-net cores: an illustration of a smart assistant-living space instrumented with various sensing devices [4].](http://oak.go.kr/repository/journal/11646/E1EIKI_2012_v6n1_67_f002.jpg)