Ultraviolet (UV)-C (short-wavelength ultraviolet) radiation has been suggested as one of the successful disinfection prac-tices for water treatment. Therefore, UV-disinfection has become a practical solution for the safe disinfection of water.
For many years chlorination has been the standard method of water disinfection. Chlorine is used in most water treatment facilities to kill harmful micro-organisms that cause serious dis-ease in drinking water. While this certainly works, the chlorine itself causes many health problems such as asthma, cancer, fer-tility problems, heart disease, eczema and birth defects. Further-more, the smell and taste of chlorinated water is very unpleasant [1]. Also, the residuals and by products from chlorination can be toxic to aquatic life in the receiving waters. Particularly, some by-products of chlorination may be carcinogenic and may require removal in a drinking water treatment plant. It has actually been discovered that chlorination is considerably less effective in virus destruction than in killing bacteria. UV light is currently a more preferable method for water disinfection. Actually, UV-disinfec-tion has gained widespread use for municipal wastewater and more recently, interest in using UV for water reuse applications has also increased [2]. It has the following inherent advantages over all other disinfection methods: no chemical consumption, thereby eliminating large scale storage; no transportation, han-dling and potential safety hazards; low contact time; no contact basin is necessary and space requirements are thus reduced; no harmful by-products are formed; a minimum of, or no, moving parts; and high reliability and low energy requirements [3]. UV-disinfection thus solves the environmental and safety problems and is also cost-effective.
UV-disinfection of water employs low-pressure mercury lamps. The lamps generate short-wave UV radiation at 253.7 nm which is lethal to micro-organisms including bacteria, pro-tozoa, viruses, molds, yeasts, fungi, nematode eggs and algae. The mechanism of micro-organisms destruction is currently believed to be that in which UV causes molecular rearrange-ments in DNA and RNA, which in turn blocks replication [4]. The acceptance of UV-disinfection at wastewater plants treating in excess of one billion gallons daily is proof that UV is no longer an emerging technology, but rather an accepted technology to be used routinely by engineers to safeguard human health and alleviate environmental pressures. Wastewater reuse has been practiced in various forms for decades, with the United States leading the way in reuse research. It is now a major issue in the U.S., where large areas of the Western and Southern states expe-rience chronic water shortages [5].
UV water purification lamps produce UV-C or germicidal UV, with radiation of much greater intensity than sunlight. Almost all of a UV lamp's output is concentrated in a 254 nm region in order to take full advantage of the germicidal properties of this wavelength. Most UV purification systems are combined with various forms of filtration, as UV light is only capable of killing micro-organisms such as bacteria, viruses, molds, algae, yeast and oocysts such as Cryptosporidium and Giardia. UV light gen-erally has no impact on chlorine, volatile organic compounds (VOCs), heavy metals and other chemical contaminants. Never-theless, it is probably the most cost-effective and efficient tech-nology available to homeowners to eliminate a wide range of biological contaminants from their water supply. This study was therefore carried out to investigate the effectiveness of UV light for wastewater disinfection [1].
The aim of this study is firstly to evaluate the kinetics of the inactivation of certain isolates of
2.1. Types and Characteristics of Treated Wastewater Used
The treated wastewater samples used in this study were col-lected at the outlet of a pilot wastewater treatment plant (WWTP) belonging to the Water Research and Technology Center, Tunisia. The pilot WWTP is connected to the sewerage network of the city of Tunis and has a processing capacity of 150 m3 per day. It is composed of four treatment lines operating in parallel: a trick-ling filter, rotating biological discs, and a soil and lagoon optional filter. During disinfection tests, the physic-chemical characteris-tics of the treated wastewater by the trickling filter did not signifi-cantly change. The values fluctuated between 47% to 49% for UV transmission, 15 to 27 mg/L for total suspended solids (TSS), 20 to 29 mg/L for biochemical oxygen demand (BOD5) and 90 to 102 mg/L for chemical oxygen demand (COD).
2.2. Experiments in a Batch Laboratory Irradiation Device
The laboratory UV device used in this study has previously been described by Hassen et al. [6]. A low pressure UV-C lamp is used. This lamp emitted an average intensity of about 7 mW/cm2. In addition, all bacterial strains studied were cultivated to a mid-log phase at 37℃ in 20 mL of nutrient broth. Each culture was centrifuged at 5,000 rpm/min for 15 min and the pellet was washed twice with sterile distilled water. The washed pellet was resuspended in 10 mL sterile distilled water. Test organisms were then seeded separately, into 20 mL of sterile wastewater of UV transmittance of 50%, to give a viable cell count of approximately a 105 to 106 unit forming colony (UFC)/mL, the same mean count as that in the secondary wastewater suspension. The test organ-isms were then exposed to the UV-C light for various times rang-ing from 2 to 90 sec.
2.3. Bacterial Strains Selected for UV-Disinfection Study
Many pathogens are responsible for waterborne diseases. Despite the development of molecular methods, currently it is not always possible to detect comprehensively all micro-organ-isms in a water sample. Therefore, most studies in this area have mainly focused on the concentration of fecal indicator bacteria (total coliforms, fecal coliforms, and fecal streptococci in gen-eral) to estimate the population of pathogens. However, recent studies showed that the species of
2.4. The Kinetic Models Used for UV-C Inactivation
These kinetic approaches are based on experimental studies using: a laboratory disinfection device; 22 selected strains of
The integration of this expression gives:
The integration of this expression gives
Changing the logarithmic form and using a linear regression, the kinetic parameters (
When
2.5. Study of the Influence of Hydrodynamics on the UV-C Disinfection
In addition tothe kinetics of disinfection, it is well known that the performance of a UV reactor depends on the hydrodynamic behavior. To study the influence of the hydrodynamic behav-ior on the UV disinfection performance, we used a UV reactor mounted at the exit of the line of the trickling filter in the waste-water pilot plant. This plant had a total capacity of treatment of 150 m3 per day. Furthermore, according to a comprehensive approach, the hydrodynamic behavior is described by the dis-tribution of residence time and is achieved by a tracer test. The Collins-Selleck model is adopted to describe the kinetics of de-crease in the number of
In this case of disinfection of treated wastewater by UV-C, the average rate of decrease in the number of bacteria
with E (θ) = e- θ, function of distribution according to the resi-dence time of water in the UV reactor; (N/N0)b, expression of dis-infection kinetics obtained by the batch tests; Θ = t/TS, reduced time; Ts = V/Q, the average residence time in the reactor. We con-sidered at first the ideal reactor as a completely mixed and plug flow reactor. Secondly, we used the model of cascading mixers (
3.1 The Inactivation Kinetic of P. aeruginosa: UV Dose-Response
The intrinsic kinetics of bacterial inactivation as a result of exposure to UV radiation are a function of the UV-C dose, ex-pressed as the product of germicidal radiation intensity (
In this study, the curve commonly illustrating the kinetics of inactivation usually showed a significant gap between the ex-perimental points and those simulated by the model in the case of all studied strains of
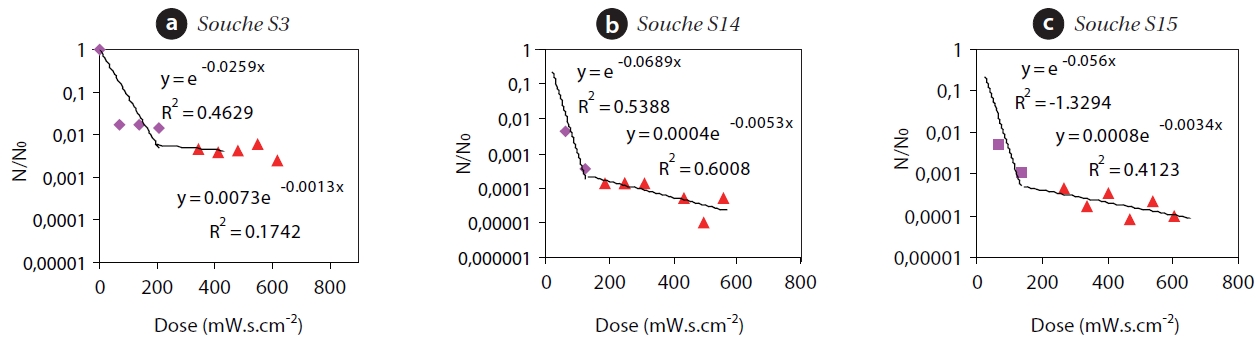
A decrease of additional U-log could not be reached, even after an exposure time of 90 sec. To improve the representative-ness of the model of Chick-Watson, in taking into account the decrease in speed during the disinfection process, the existence of two stages, each with different kinetics is realized in Fig. 1 : 1) Fast inactivation kinetics with low doses varying between 0 and 200 mW/sec/cm2 and a coefficient of lethality ranging between -0.0259, -0.0689, and -0.056 for strains S3, S14, and S15, respec-tively, taken as an example. This result is confirmed by the work of [17] and [18] concerning the inactivation of bacillus spores by UV rays. 2) Slow kinetics with doses ranging between 200 and 600 mW/sec/cm2, and a coefficient of relatively low lethality between -0.0012, -0.0053, and -0.0034, respectively for the same three strains. This result has been described by several authors [19-21]. It is therefore necessary to assume the existence of at least two stages during the inactivation process of which only the second was explored during these tests.
The application of a first order kinetic during the second
The kinetics characteristics of all the disinfection models studied during ultraviolet (UV) irradiationa
The kinetics characteristics of all the disinfection models studied during ultraviolet (UV) irradiationa
stage requires the modification of the model by introducing a di-mensionless coefficient
with
In the same way, passing to the logarithm scale, the expression becomes:
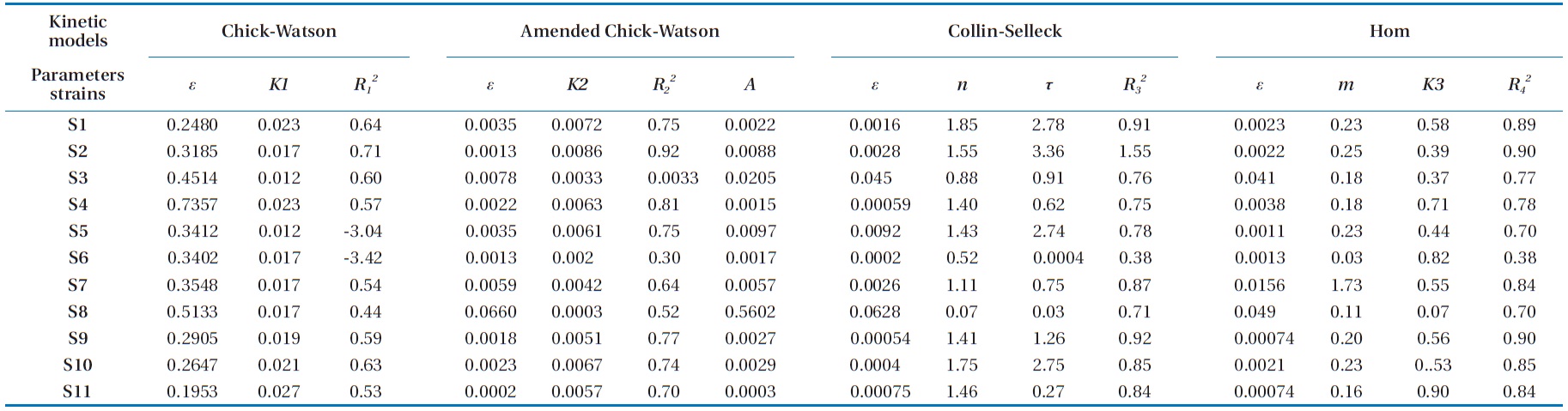
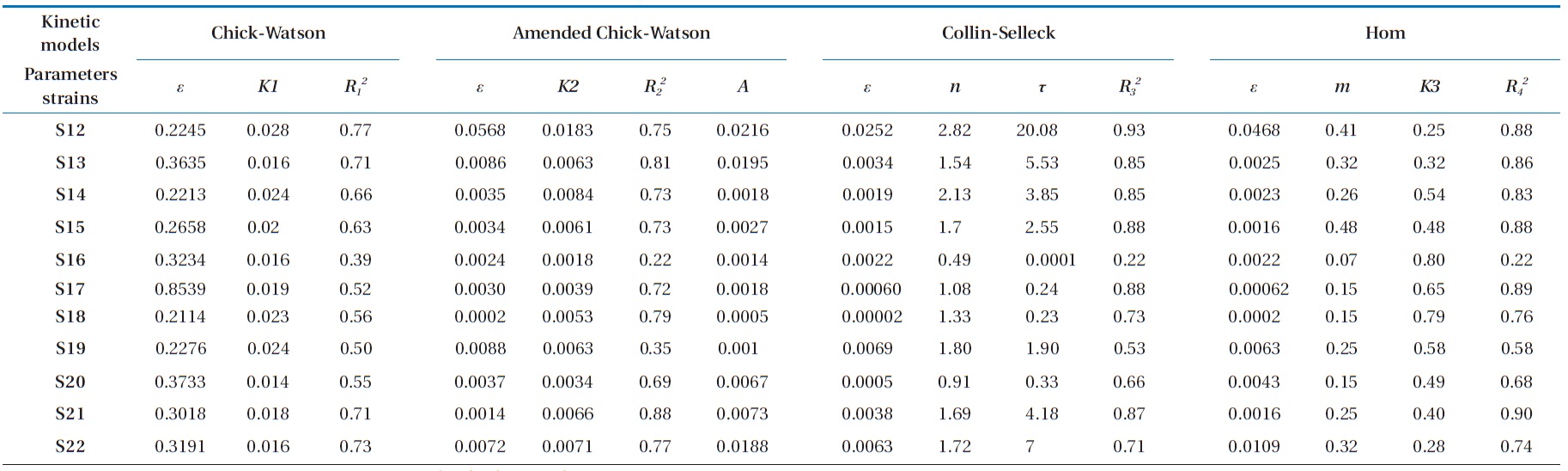
We can determine the kinetic equations and the coefficient of reliability of the model for each strain studied using a linear regression. The kinetic parameters obtained of this modified model (
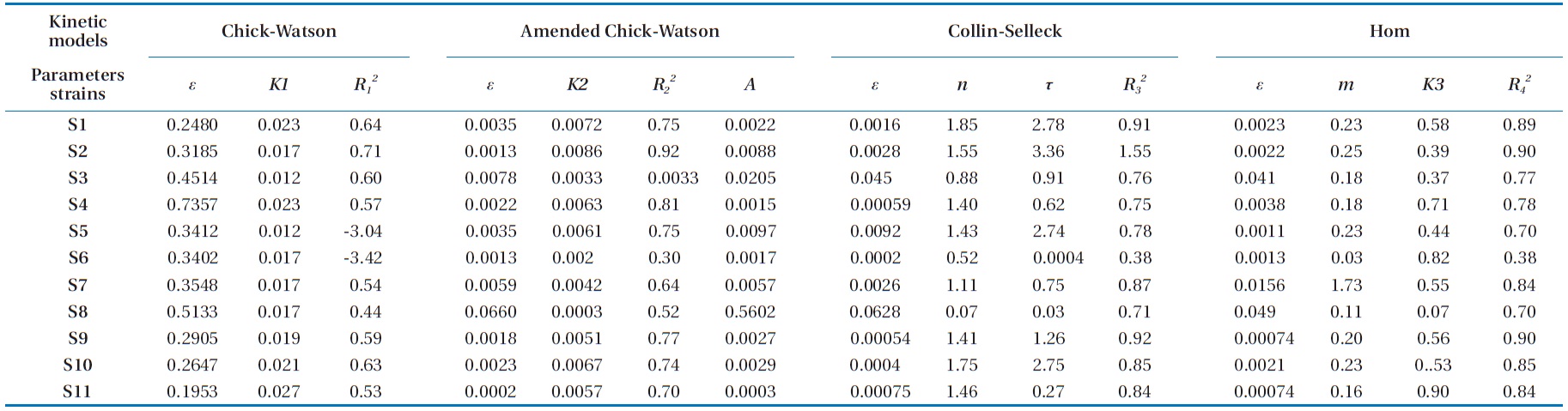
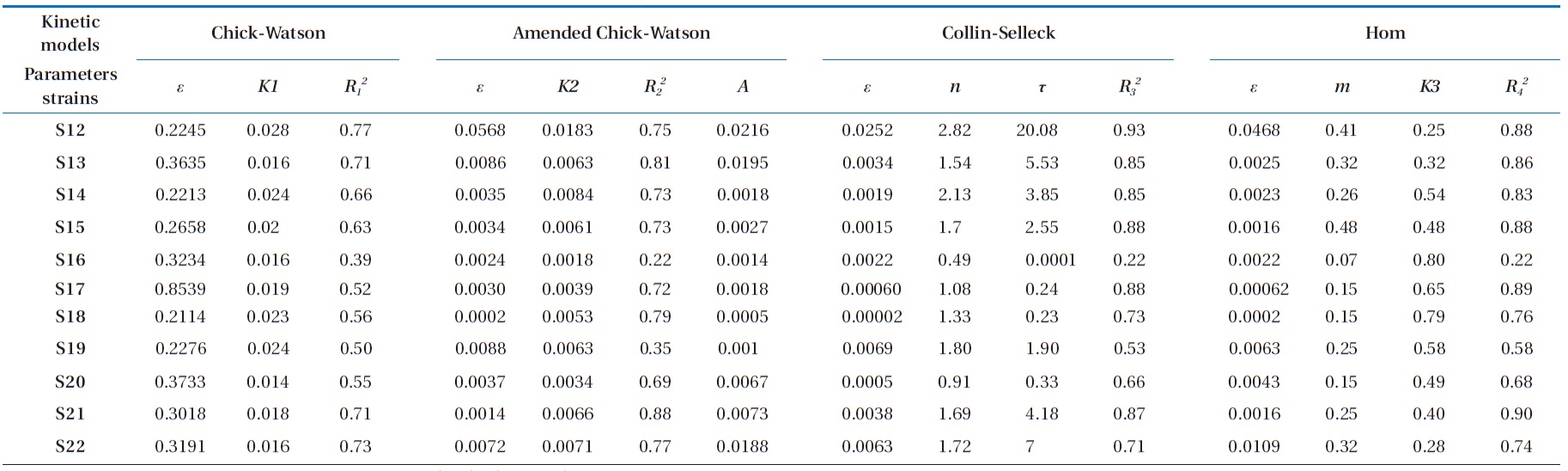
Referring to the results of the kinetic parameters of the mod-el summarized in Tables 1 and 2, we can deduce a remarkable similarity between the values of the kinetic constant
1) If 0002 ≤
2) If 0005 ≤
3) Group 3 includes strains S14 and S22 with a
By calculating the difference
for these two models, the values obtained depending on the model of Chick-Watson in its modified form were smaller than those calculated using the same model in its initial form. In the same way, the coefficients of determination
A key feature of kinetic modeling is not only its simplicity but also that it idealizes a complex phenomenon of disinfection systems. Observation and mathematical modeling of microbial inactivation provides indirect information on the physiological mechanism of inactivation, and equally the mechanisms of re-sistance.
Several models have been proposed to explain the kinetics of inactivation resulting from the existence of the latency period following the contact of water and disinfectant [22-26]. During this period of latency, the decrease rate of bacteria number is not measurable. This was observed for
In UV-disinfection, several models, for example, the model of Collins-Selleck [24], the Series event model [30] and the multi-shock model [30, 31] have been developed to describe the initial plateau observed when micro-organisms are exposed to a sub le-thal UV dose. In this case, bacterial inactivation is not significant and the bacteria decline is of low amplitude [20, 32-34].
This latency stage of inactivation for certain strains of
τ is the least dose of radiation to be reached to start the process of micro-organism inactivation;
In some cases, as mentioned above, laboratory results showed that the disinfection law proposed by the model of Chick-Watson was problematic in simulating the experimental data. The study of [36] on the inactivation of a strain of
Integrating this Equation for the constant
where
In the Hom model, if the parameter
In the case of UV-disinfection, the factor
When
the parameters to be identified in this case are
and using a linear fit, we can determine the different values of
Similarly, the determination of
The Hom model of the form:
describes fairly well the kinetics of disinfection.
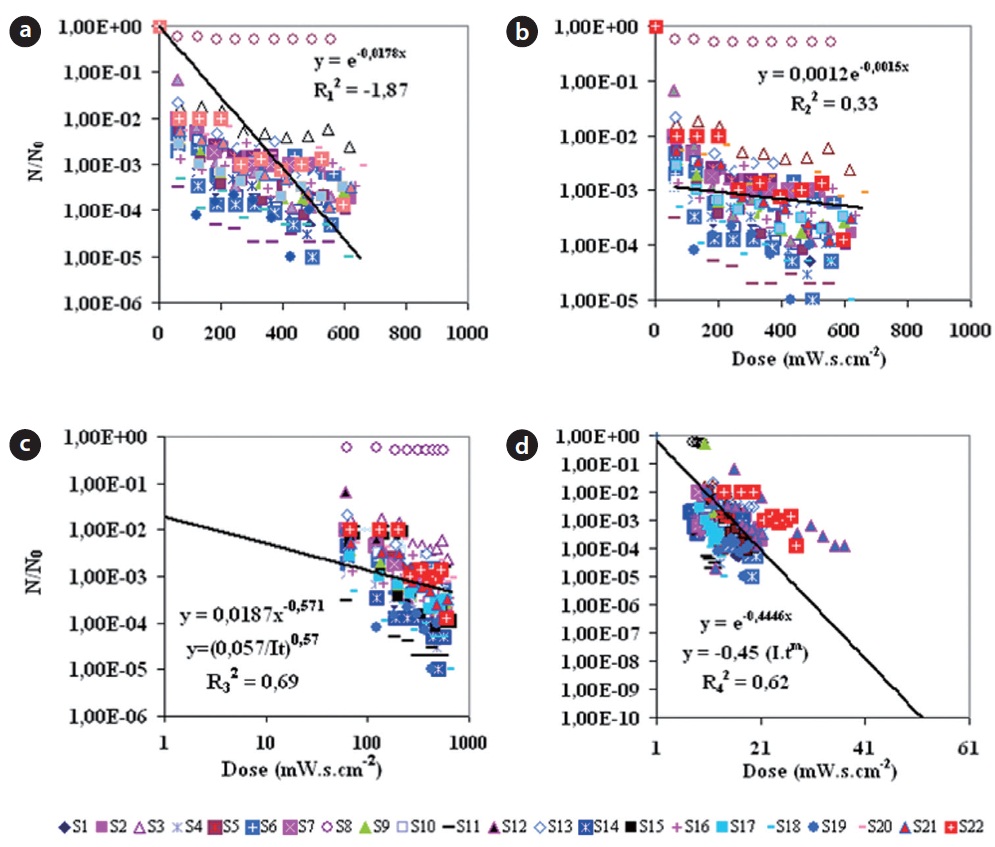
For an overall approach in Fig. 2, and for all regression models, the correlation coefficients were respectively -1.86 for the origi-nal Chick-Watson model, 0.32 for the amended Chick-Watson model, 0.62 for the Hom model, and 0.69 for the Collins-Selleck model. However, even an
In this regard, the determination of
3.2. Influence of Hydrodynamics on the Performance of the Disinfection
To study the hydrodynamic influence on the UV reactor per-formance, we used treated wastewater at the exit of the line of the trickling filter in the pilot plant, and we considered at first the ideal reactor as a completely mixed and plug flow reactor. Secondly, we used the model of cascading mixers (
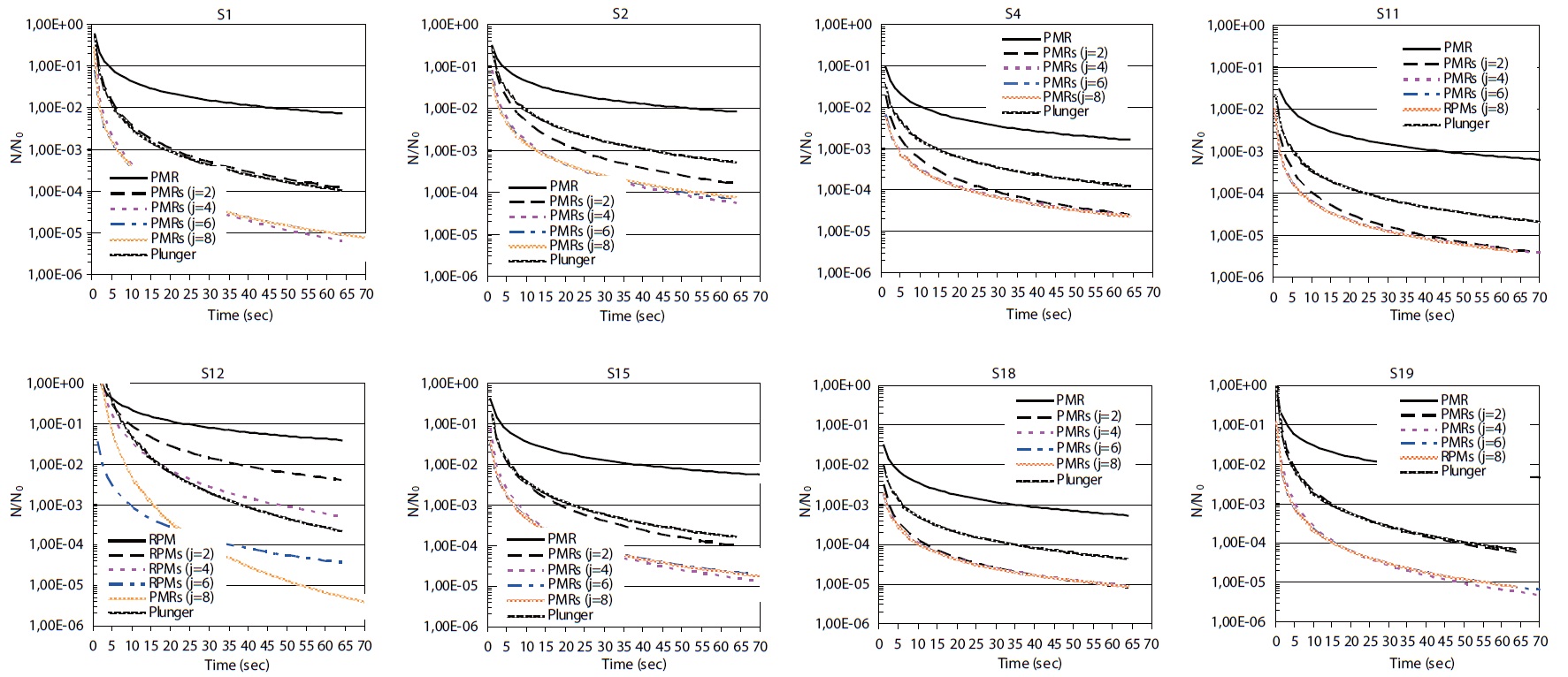
Numerical computing and integration of all functions that ex-press the rates of decline for the 8 strains of
In our approach and in order to model the hydrodynamic flow in the reactor and the short distance between the outlet of the trickling filter and the UV reactor, the average residence time in the irradiation room of the UV reactor is calculated experi-mentally using the time of passage theoretically observed (10, 20, 30, 40, 50, 60, 70, 80, and 90 sec) and for all flows tested without resorting to the technique of chemical tracing.
In a plunger reactor, there is no distribution of the residence time. The batch kinetics is sufficient to give the performance of disinfection:
3.4. Case of Perfectly Mixed Reactor (j=1)
In UV-disinfection and for all the strains studied, we use the Collins-Selleck model to express the kinetics of disinfection in a closed reactor. We can therefore write:
where τ is kinetic parameter of the model of Collins-Selleck;
3.5. Model of Cascading Mixers (j > 1)
In a closed reactor, if we combine the hydraulic model of
where τ is kinetic parameter of the model of Collins-Selleck;
Several standardized international guidelines stipulate that the reuse of wastewater requires a decrease in the number of fe-cal coliforms of about 3-log. However, the complexity of current processes and requirements for environmental safety, microbi-ology, public health and even industry, require the introduction of advanced monitoring systems based on monitoring method-ologies built on the principle of analytical redundancy. For this reason, a second standard requires a reduction ratio of the num-ber of
In the same process, if we consider that our UV reactor would operate as a plug flow reactor, in this case an improvement of the disinfection process has been observed for some
on the inactivation rate for the other strain, which failed to reach 10-4, the performance required by several standardized interna-tional guidelines if purified water is reused for agricultural pur-poses. Similarly, we can deduce here that the nature of flows in the reactor has more impact on the final yield of disinfection. We can see for example in Fig. 3 (strain S19), a plug flow reactor is 14 times more efficient than a perfectly mixed reactor where the residence time is 40 sec. This divergence is even more prominent for higher residence times, and is thus most important for UV doses, and this result is valid for strain S12 taken as an example.
A significant improvement in the microbiological water qual-ity is observed when the UV reactor operates as two perfectly mixed reactors in series. This is noticed mainly for strains S1, S18, and S19 where a removal efficiency of 4-log is figured de-spite the wide divergence in their responses to residence time as discussed. Indeed, a residence time of 27 sec appeared sufficient to meet the standards required for the S4 strain; on the contrary, to reach the same performance, a time of residence of 10 and 50 sec is required for strains S11 and S19, respectively.
With regard to the major observations advanced earlier and to better meet the requirements of environmental microbiologi-cal safety, public health and even industry, seeking other alter-natives to replace the first simulation that has shown its limits seems to be indisputable, despite the improvement of the rate of efficiency registered for some strains. If we assimilate our UV-C reactor by a succession of reactors in series (
A succession of 6 or even 8 perfectly mixed reactors in series do not significantly improve the efficiency of UV-disinfection for almost all strains examined and with residence times of up to 70 sec. In addition, Figs. 1 and 2 taken as a model showed that the removal rate of bacteria if the reactor operates as a succession of 6 and even 8 perfectly mixed reactors in series seemed to be a combination of 4 reactors placed in series. With respect to all interpretations that we advanced for the process of UV-disinfec-tion of treated wastewater, in order to assume a complete inactivation of
The application of the original model of Chick-Watson was not sufficiently representative to describe the kinetics of bacte-rial inactivation. Therefore, a modification based on the same model, but after taking into consideration an initial inactivation described, very well the kinetics of disinfection. According to pa-rameter
Finally, in considering all the interpretations advanced previ-ously concerning the process of disinfection of treated wastewa-ter by UV radiation and for a complete microbial cleaning, we need to simulate the operation of the UV reactor as a succession of four perfectly mixed reactors in series.