The eutrophication of lakes and seas by phosphorus is a widespread environmental problem, posing a great threat to aquatic environments. Recently, considerable attention has been paid to the use of layered double hydroxides (LDHs) for phosphate removal because of their high surface area and large anion exchange capacity [1]. LDHs are a class of nanostructured anionic clays. They have a general formula of [M1-x2+Mx3+(OH)2] x+(An-)x/n·mH2O, where M2+ is the divalent cation, M3+ is the trivalent cation, x is the molar ratio of M3+/(M2++M3+), and A is the interlayer anion of valence n. LDHs consist of positively charged brucite-like sheets which are balanced by the intercalation of anions in the hydrated interlayer regions [1]. Several researchers have examined the phosphate removal by various LDHs including Mg-Al LDHs [2, 3], Zn-Al LDHs [4-6], Mg-Mn LDHs [7], and iron-based LDHs [8]. They have demonstrated that LDHs could be applicable to phosphate removal.
Due to low hydraulic conductivity and large sludge production, however, a powder form of LDHs might not be suitable in wastewater treatment systems. Thus, the granular forms of LDHs have been synthesized by few researchers, including Mg-Al LDHcoated sand [9], Mg-Al LDH aggregates [10], Mg-Al LDH-coated zeolites [11], and granular Mg-Al LDH [12]. The entrapment of functional materials into calcium alginate beads has been widely applied in regards to water treatment because it is a simple and cost effective technique [13-17]. Sodium alginate is composed of (1-4)-linked D-mannuronic and L-glucuronic acid repeat units. Calcium ions can chelate carboxylate groups, make crosslinks between chains, and form an insoluble hydrogel [18]. However, the mechanical strength and chemical stability of alginate hydrogel is poor. It is, therefore, necessary to improve its properties by blending it with polymers such as polyvinyl alcohol (PVA) [14].
In this study, LDH-PVA/alginate beads were synthesized through immobilization of Mg-Al LDH into calcium alginate beads blending with PVA. The properties of LDH-PVA/alginate beads were analyzed using a field emission scanning electron microscopy (FESEM) combined with an energy dispersive X-ray spectrometer (EDS). The stability of LDH-PVA/alginate beads in a phosphate solution was compared with LDH-alginate beads. Also, the removal of phosphate in LDH-PVA/alginate beads was examined using batch and column experiments.
2.1. Synthesis of LDH-PVA/alginate Beads
All chemicals used for the experiments were purchased from Sigma Aldrich. A powdered form of LDH was prepared by coprecipitating mixtures of magnesium nitrate [Mg(NO3)2·6H2O] and aluminum nitrate [Al(NO3)3·9H2O] following the procedures described in [19]. The Mg-Al LDH was obtained via calcination at 573 K for 24 hr in an electric muffle furnace (C-FMA; Vision Lab, Seoul, Korea). LDH-PVA/alginate beads were prepared by entrapping a powdered form of Mg-Al LDH in a PVA and a calcium alginate blend gel. Twenty grams of PVA and two grams of sodium alginate were dissolved in 200 mL of distilled water and agitated on a magnetic stirrer at 70℃ for 5 hr. Then, 16 g of LDH was added to 200 mL PVA/alginate solution under intensive stirring to obtain the homogeneous suspension. The suspension contained in plastic syringe (outlet diameter, 2 mm) was allowed to drop into a stirred reservoir containing a 500 mL solution of 0.3 M calcium chloride (CaCl2) in order to form 4.20 ± 0.25 mm (number of beads, 90) spherical beads (LDH-PVA/alginate beads). The beads were allowed to cure in the same CaCl2 solution for 24 hr under stirring and then rinsed with deionized water to remove excess Ca2+. In addition, LDH-alginate beads were prepared by entrapping a powder form of Mg-Al LDH into a calcium alginate gel following the same procedures used for LDH-PVA/ alginate beads excluding PVA.
2.2. Characterization and Stability of LDH-PVA/alginate Beads
LDH-PVA/alginate beads were examined by X-ray microanalysis, as described in the literature [20], briefly, specimens were mounted on metal stubs using carbon tape and sputtercoated with platinum (approximately 20 nm in thickness). Specimens were examined with a FESEM (Supra 55VP; Carl Zeiss, Oberkochen, Germany) at an accelerating voltage of 3 kV. In addition, the elemental composition was determined with EDS (XFlash 4000; Bruker AXS, Berlin, Germany), combined with an electron microscope at an accelerating voltage of 15 kV. X-rays were collected with a detector fixed at a take-off angle of 35°, and region analysis and mapping were performed on the specimens.
The stability of LDH-alginate beads and LDH-PVA/alginate beads in a phosphate solution was compared under various phosphate concentrations (50, 100, 150, 200 mgP/L). Both beads (numbers of beads, 51?53) were added to a 30 mL phosphate solution in 50 mL polypropylene conical tubes. The tubes were shaken at 25℃ and 40 rpm using a culture tube rotator (MG- 150D; Mega Science, Seoul, Korea). After 24 hr, solutions were poured into petri dishes, and then the numbers of beads with a distinct shape were counted.
All experiments were performed at the solution pH of 4.9 (excluding the pH effect tests). Kinetic batch experiments were performed with PVA/alginate beads and LDH-PVA/alginate beads at the initial phosphate concentration of 25 mgP/L. The desired phosphate solution was prepared by diluting the stock solution (1,000 mgP/L), which was made from potassium dihydrogen phosphate (KH2PO4). Two grams of beads were added to a 30 mL phosphate solution in 50 mL polypropylene conical tubes. The tubes were shaken at 25℃ and 40 rpm using a culture tube rotator (MG-150D; Mega Science, Seoul, Korea). In the experiments, the samples were taken at 1, 2, 3, 4, 5, 6, 12, and 24 hr after the reaction and filtered through a 0.45 μm membrane filter. The phosphate was analyzed by the ascorbic acid method [21]. Phosphate concentration was measured at a wavelength of 880 nm using a UV-vis spectrophotometer (Helios; Thermo Scientific, Waltham, MA, USA).
Equilibrium batch experiments were conducted with LDHPVA/ alginate beads (2 g) at different initial phosphate concentrations. Samples were collected 12 hr post-reaction. Further batch experiments were performed to observe the effect of solution pH on phosphate removal in LDH-PVA/alginate beads. The experiments were conducted at an initial phosphate concentration of 25 mgP/L with 2 g of LDH-PVA/alginate beads. The initial solution pHs ranged from 4.9 to 8.9. The pH was measured with a pH probe (9107BN; Thermo Scientific). All experiments were performed in triplicate.
Three column experiments were performed using a Plexiglas column (inner diameter, 2.5 cm; bed depth, 10 cm; bulk volume, 49.1 cm3) packed with PVA/alginate beads, LDH-PVA/alginate beads, and LDH-alginate beads. Prior to the experiments, the packed column was flushed upward using a HPLC pump (Series II pump; Scientific Systems Inc., State College, PA, USA) operating at a rate of 0.5 mL/min with 5 bed volumes (BV) of deionized water until a steady state flow condition was established. Then, a phosphate solution (10 mgP/L) was introduced downward to the packed column at the same flow rate. The empty bed contact time was determined to be 26.5 min. Portions of the effluent were collected using the auto collector (Retriever 500; Teledyne, Lincoln, NE, USA) at regular intervals, and phosphate was analyzed by the ascorbic acid method [21].
3.1. Stability and Characteristics of LDH-PVA/alginate Beads
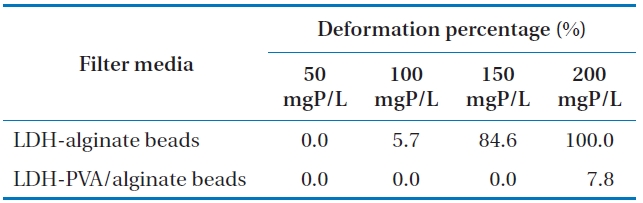
The deformation of LDH-alginate beads and LDH-PVA/alginate beads at various phosphate concentrations under batch condition are demonstrated in Fig. 1. The deformation percentages (
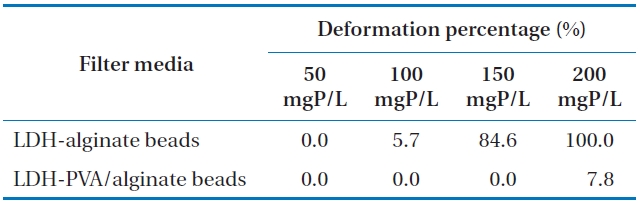
Deformation of LDH-alginate beads and LDH-PVA/alginate beads under different phosphate solutions
where
The FESEM image and EDS pattern for LDH-PVA/alginate beads are presented in Fig. 2. It demonstrated that the crosssectional surface of LDH-PVA/alginate beads was heterogeneous in surface topography in Fig. 2(a). The powders of LDH were intermingled with PVA/alginate polymers. The sheet-like polymer matrices were present on the cross-sectional surface of LDH-PVA/alginate beads in Fig. 2(b). Calcium and chloride were evident at the peak positions of 3.69 and 2.62 keV as K-alpha Xray signals, respectively. In addition, magnesium and aluminum were found at 1.25 and 1.48 keV as K-alpha X-ray signals, respectively in Fig. 2(c).
3.2. Phosphate Removal in Batch Condition
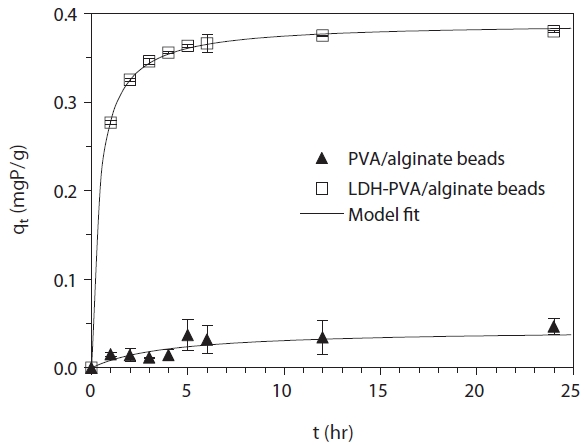
The kinetic data and model fit for phosphate removal in PVA/ alginate beads and LDH-PVA/alginate beads are presented in Fig. 3. It revealed that the reaction reached equilibrium after 12 hr of reaction time. In PVA/alginate beads, the average removal percentage was 12.1% while it was 99.2% in LDH-PVA/alginate beads, indicating the sharp increase of phosphate removal in the presence of LDH. The kinetic data were analyzed by the pseudo second-order model [25]:
where
beads. The value of
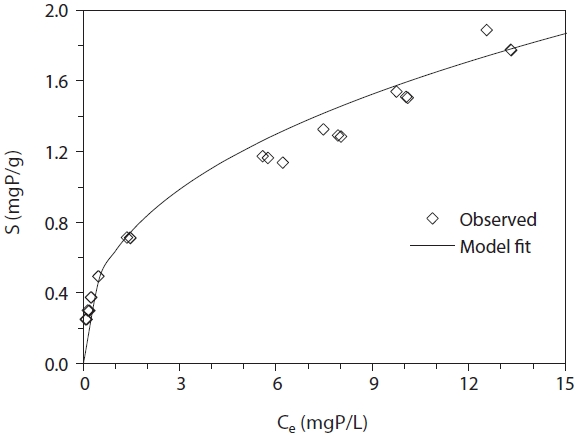
The equilibrium data and model fit for phosphate removal in LDH-PVA/alginate beads are given in Fig. 4. The data were analyzed by the Freundlich isotherm model:
where
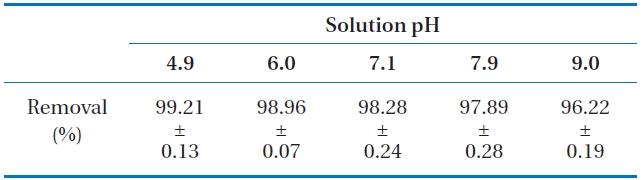
The influence of solution pH on phosphate removal in LDHPVA/ alginate beads is presented in Table 2. The average removal percent was 99.21% at pH 4.9, decreasing slightly to 96.22% with the solution pH approaching 9.0. This indicated that phosphate removal in LDH-PVA/alginate beads was not sensitive to pH change for the given experimental conditions. Our result did not conform with the report of Das et al. [19] demonstrated that phosphate removal in the powder form of Mg-Al LDH sharply decreased from 91.7% to 39.0% with increasing pH from 5.0 to 10.0.
3.3. Phosphate Removal in Flow-Through Column Condition
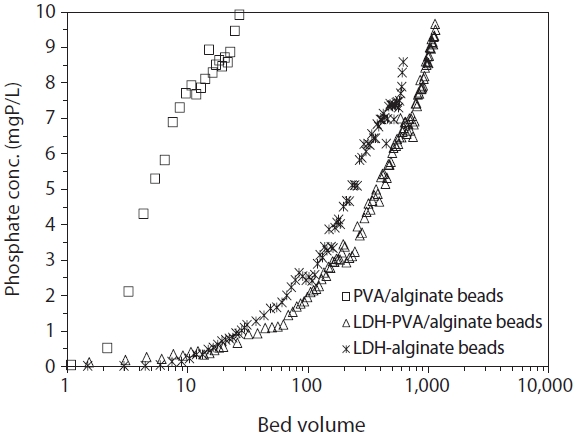
The phosphate breakthrough curves (BTC) in PVA/alginate beads, LDH-PVA/alginate beads, and LDH-alginate beads are presented in Fig. 5. The breakthrough data was presented in terms of phosphate concentration versus BV. At the given flow conditions, the BTC for PVA/alginate beads increased sharply, reaching 95% of the initial phosphate concentration (9.5 mgP/L) around 25 BV. For the same conditions, the BTCs for LDH-PVA/ alginate beads and LDH-alginate beads were distinctively delayed relative to that for PVA/alginate beads. In LDH-PVA/alginate beads, the BTC increased slowly, reaching to
The column capacity for phosphate removal (
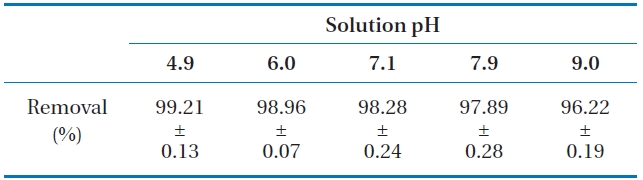
Phosphate removal in LDH-PVA/alginate beads under different solution pH (initial phosphate concentration, 25 mgP/L)
tified using the following equation [26]:
where
where
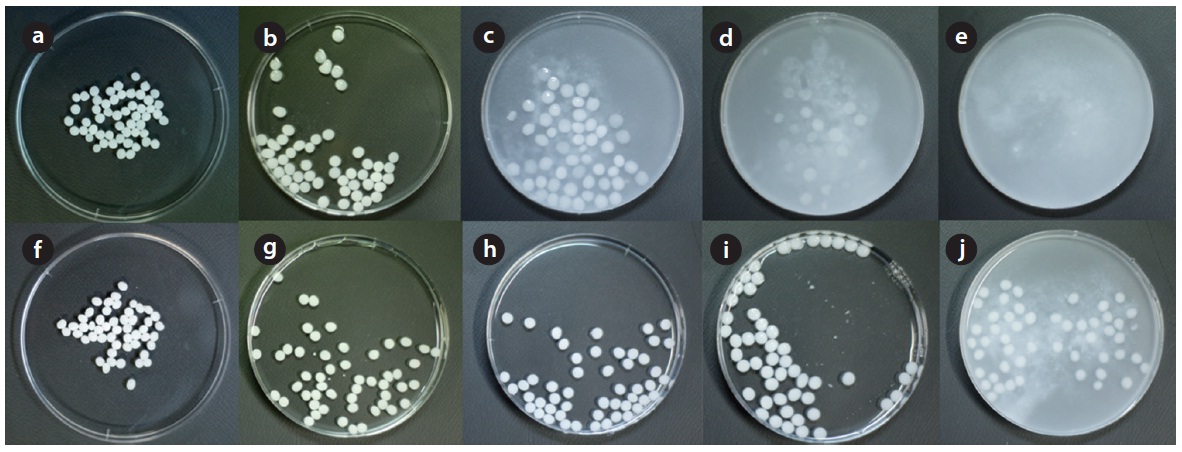
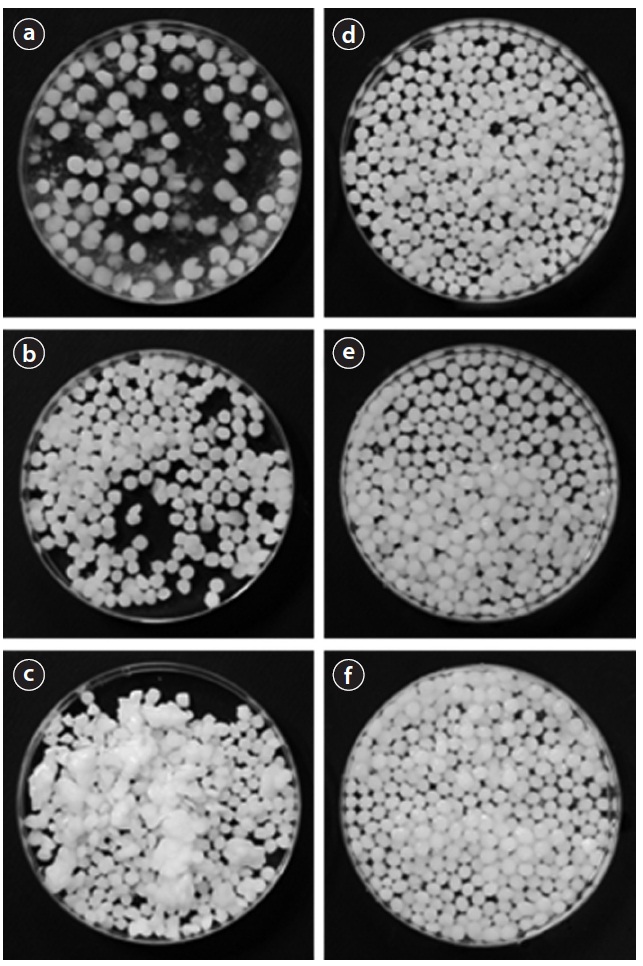
It should be noted that the column experiment in LDH-alginate beads was discontinued around 620 BV due to the bead deformation observed at the top of the column. After the column experiments, the beads were taken out from the column and then observed for the effect of phosphate solution on their stability. As shown in Fig. 6, the deformation of LDH-alginate beads Fig. 6(a-c) was notable, especially on top of the column. However, the deformation of LDH-PVA/alginate beads Fig. 6(d-f) was not eminent throughout the column. It indicated that blending PVA with calcium alginate beads considerably improves the stability of the beads during phosphate removal.
In flow-through column experiments, phosphate ions could move through the paths formed among the beads and diffuse into the beads through the pores in the beads. Then, the phosphate could come in contact with the entrapped LDH particles and subsequently be removed from the solution via three different mechanisms. First, the negatively-charged phosphate could adsorb to the positively-charged brucite-like layer in surface adsorption. Second, the phosphate could replace the charge balancing anion (carbonate) in the interlayer region in the anion exchange process. Third, the phosphate could intercalate into the interlayer due to the reformation effect of the calcined LDH in the aqueous phase [1, 5, 6].
In this study, LDH-PVA/alginate beads were synthesized and used for phosphate removal. Results showed that blending PVA with the LDH-alginate beads significantly improved their stability in the phosphate solution. The results also indicated that the phosphate removal reaction in LDH-PVA/alginate beads reached equilibrium at 12 hr-post reaction with a high removal percentage. The equilibrium batch data were well described by the Freundlich isotherm model. In addition, phosphate removal in LDH-PVA/alginate beads was not sensitive to solution pH. Furthermore, results also showed that the removal capacity of
LDH-PVA/alginate beads in the column experiments was two orders of magnitude greater than that of PVA/alginate beads. This study demonstrates that LDH-PVA/alginate beads possess higher chemical stability against phosphate than LDH-alginate beads and have the potential for phosphate removal as adsorptive media.