Carotenoids belong to the tetraterpenes family and are found in plants, algae, photosynthetic bacteria and marine ani-mals. The distribution of carotenoids in marine animal sources is primarily the result of specific dietary habits, absorption, and metabolic transformation (Hosokawa et al., 2009). A high level of carotenoids was isolated from the tunicate
Carotenoids serve a protective role by effectively dissipat-ing excess energy, preventing the formation of reactive oxygen species (ROS), and by deactivating singlet oxygen molecules generated during the photosynthetic process (Chew and Park, 2004; Jackson et al., 2008). In addition, dietary carotenoids re-act with a wide range of free radicals such as CCl3O2·, RSO2·, NO2· and various arylperoxy radicals via electron transfer, pro-ducing the radical cations of the carotenoids. DPPH radicals and the 2, 2′-azinobis-3-ethylbenzotiazoline-6-sulphonic acid (ABTS) radical scavenging assay are popular indirect meth-ods of determining the antioxidative capacity of compounds. The DPPH radical scavenging activities of fucoxanthin and fucoxanthinol were higher than of halocynthiaxanthin, with concentrations (μM) required for 50% scavenging (EC50) of 164.6, 153.8 and 826.4, respectively. Additionally, ABTS radical scavenging activity of fucoxanthinol (EC50-2.49 μM) was stronger than that of fucoxanthin (EC50-8.94 μM). Fur-thermore, the hydroxyl radical scavenging activity, as mea-sured using the chemiluminescence technique, showed that the scavenging activity by fucoxanthin was 7.9 times higher than that of fucoxanthinol, 16.3 times higher than that of halo-cynthiaxanthin and 13.5 times higher than that of α-tocopherol (Shon et al., 2003).
The objectives of this study were to evaluate and compare the effect of antioxidant properties of mideodeok muscle and tunic harvested from two different areas in the southern coast of Korea. Specifically, we aimed to provide baseline informa-tion on the antioxidant capacity of mideodeok for both con-sumers and researchers working on ascidians, which could provide insight into more extensive use of this marine organ-ism, particularly as food material.
Samples of
Carotenoids were extracted from mideodeok muscle and tunic with acetone at room temperature. After concentration under reduced pressure, the carotenoids were transferred to diethyl ether by the addition of deionized water. The resultant carotenoids were quantified using a UV Spectrophotometer (UV-1700; Shimadzu Co., Kyoto, Japan). The absorbency de-tection wavelength was set at 460 nm and the analyses were conducted in triplicate (McBeth, 1972). The total carotenoids content was calculated using the following equation: total carotenoids (mg/kg) = [O.D. (λmax) × Vol. × 1,000]/ [E1%1cm (2,400) × weight of sample (kg)]
Total phenolic content (TPC) was determined according to the Folin-Ciocalteau method (Slinkard and Singleton, 1977) with slight modifications. Briefly, 100 μL of carotinoid extract (0.12%, w/v in ethanol) was mixed with 1.4 mL deionized wa-ter and 100 μL Folin-Ciocalteu reagent (Sigma-Aldrich Co., St. Louis, MO, USA). The solution was vortexed for 30 s. So-dium carbonate solution (20%, w/v) was added to the mixture and vortexed for another 30 s. The mixture was allowed to stand at room temperature for 2 h, after which the absorbance was measured at 765 nm against a blank sample. A standard calibration curve was made using different concentrations of gallic acid (Sigma-Aldrich Co.). The concentration of TPC in the sample was expressed as mg gallic acid equivalent (GAE) of extract.
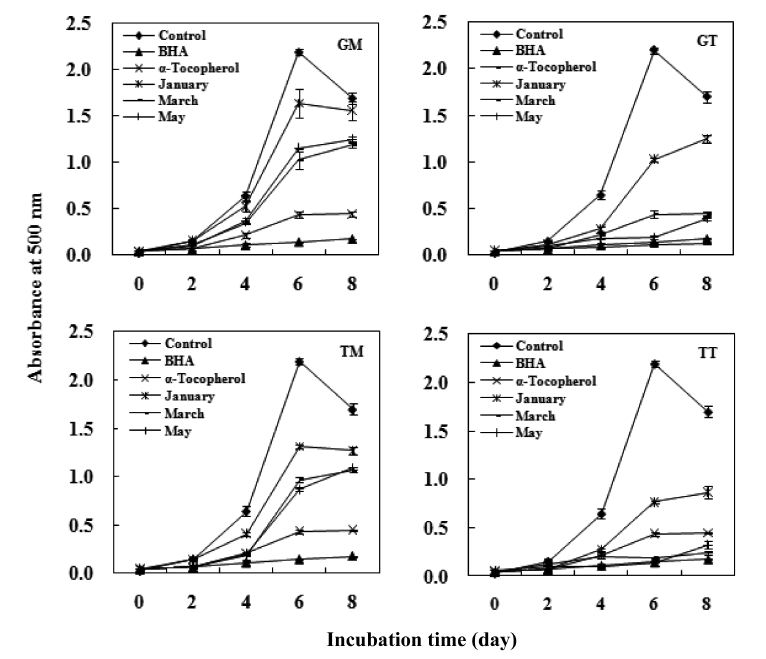
The antioxidant activity of the carotenoids was measured by linoleic acid peroxidation and was quantified using the ferric thiocyanate method (FTC) developed by Osawa and Namiki (1981). A 4 mL aliquot of sample (0.12%, w/v in ethanol) was mixed with 4 mL linoleic acid (2.5%, v/v) in 99.5% ethanol, 8 mL phosphate buffer (pH 7.0, 0.05 M) and 3.9 mL deion-ized water. The mixture was kept in a screwed-cap vial and incubated at 40℃ under dark conditions until the day after the absorbance of the control reached a maximum. α-Tocopherol and butylated hydroxyanisole (BHA) were prepared under the same conditions as the positive standards. The control was prepared in the same way but without the test compound. FTC analysis was carried out at 2 day intervals.
>
DPPH radical scavenging activity
The free radical scavenging activities of the carotenoids were measured using the DPPH method modified after Oyaizu (1986). A 0.5 mL aliquot of the 0.5 mM DPPH-ethanol so-lution was added to 1 mg/mL of carotenoids (0.12%, w/v in ethanol). Next, 1 mL of ethanol was added to the mixture and the volume was adjusted to 2.5 mL with 0.1 M sodium acetate buffer (pH 5.5). α-Tocopherol and BHA were prepared under the same conditions as the positive standards. The mixture was shaken vigorously and left at room temperature for 30 min. The absorbance was measured at 517 nm.
>
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity of the carotenoids was evaluated using the 2-deoxyribose oxidation method (Chung et al., 1977) with slight modifications. Specifically, 0.2 mL of 10 mM 2-deoxyribose, 0.2 mL of 10 mM Fe2+/EDTA and 0.2 mL of 10 mM H2O2 were added to 10, 30, 50, and 100 μL of carotenoids (0.12%, w/v in ethanol). The final volume, adjusted to 2 mL with 100 mM phosphate buffer solu-tion (pH 7.4) and the reaction mixture, was incubated at 37℃ for 4 h. After incubation, 1 mL of each of 2.8% trichloroace-tic and 1% thiobarbituric acid in 50 mM NaOH were added and the mixture was heated at 100℃ for 10 min, cooled in an ice bath, and the absorbance was measured at 532 nm. The hydroxyl radical scavenging activity was calculated using the following equation:
hydroxyl radical scavenging activity (%) = [1- (Sample Abs/Control Abs)] × 100
The reducing powers of the carotenoids were determined using the method described by Oyaizu (1986). Briefly, a sam-ple solution was mixed with 0.5 mL of 0.2 M phosphate buffer (pH 6.6) and 0.5 mL of potassium ferricyanide (III) (1%, w/v) followed by a 50℃ incubation for 20 min. Next, 0.5 mL TCA (10%, w/v) was added to the mixture before centrifuging at 1,036 g for 10 min. A 0.5 mL aliquot of the upper layer of the solution was then mixed with 0.5 mL distilled water and 0.1 mL FeCl3 (0.1%, w/v), after which the absorbance was mea-sured at 700 nm. A higher absorbance was taken to indicate a greater reducing power.
Data were evaluated for statistical significance using the JMP Statistical Discovery Software™ version 5 (SAS Insti-tute Inc., Cary, NC, USA). Values were expressed as the mean ± SD. The mean values were compared using a one-way anal-ysis of variance (ANOVA) followed by Tukey’s or Duncan’s test. A
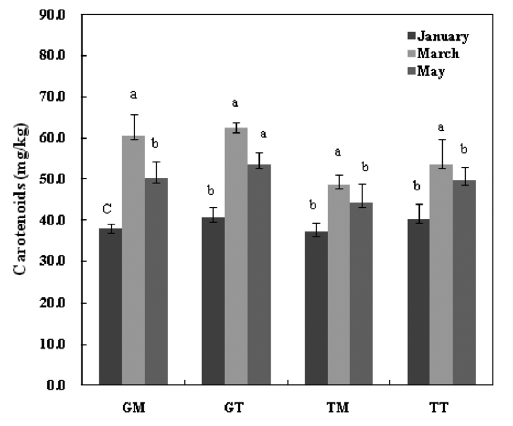
The total carotenoid contents of the
dietary antioxidants, given their ability to counteract oxidative damage to biomolecules and protect against chronic diseases such as cancer, cardio-vascular diseases and visual degenera-tion in humans (Kelly et al., 1993). They also act as precursors for vitamin A by means of β-carotene. Thus, mideodeok may be a good alternative food for humans to provide the carot-enoids necessary for maintaining health.
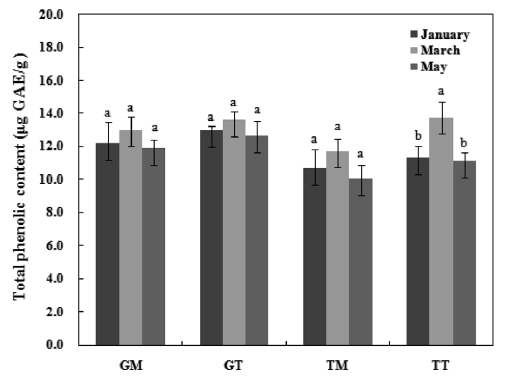
The TPC of mideodeok muscle and tunic carotenoids are shown in Fig. 2. The data are expressed as μg GAE/g carot-enoids. The Geoje sample exhibited higher phenolic levels
than the Tongyeong sample. Furthermore, the TPC in the muscle was lower than that of the tunic extract. However, no significant differences were detected among samples, with the exception of TT. The TPC levels in GM, GT, TM, and TT ranged from 11.9-13.0 GAE μg/g, 12.6-13.6 GAE μg/g, 10.0-11.7 GAE μg/g, and 11.1-13.8 GAE μg/g, respectively. Lee et al. (2010) extracted active compounds from
A considerable number of studies have provided
>
DPPH radical scavenging activity
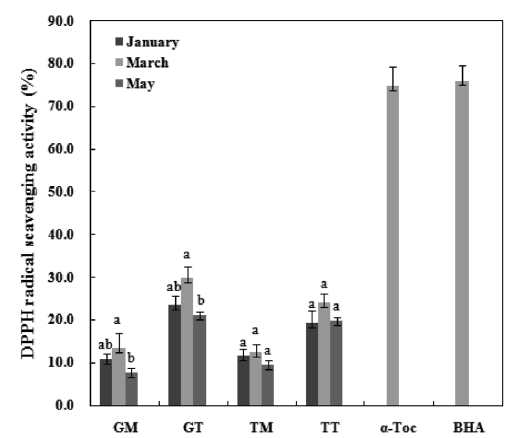
DPPH is a free radical compound and has been widely used to test the free radical scavenging abilities of various samples (Roginsky and Lissi, 2005). The result of the DPPH radical scavenging activity analyses are presented in Fig. 4. The final concentrations of α-tocopherol and BHA used were 0.1 mg/mL while those of the mideodeok carotenoids were 1.0 mg/mL. The activities of the carotenoids were very low compared to the standards. The GT March sample showed the highest ac-tivity among extracts, with a capability of scavenging 29.9% of the 5 mM DPPH radical for 30 min. The muscle samples showed low activity at 7.6-13.5% in the GM and 9.6-12.4% in the TM. However, the standards showed greater than 75% of the DPPH radical scavenging effects at low concentrations. A positive correlation was observed between phenolic content (Fig. 2) and DPPH radical scavenging activity. Previous stud-ies have suggested that the DPPH radical scavenging capaci-ties of extracts are largely affected by the presence and posi-tion of the phenolic hydroxyl group. The anti-radical activity of the phenolic compound is, in turn, dependent on its mo-lecular structure, i.e., the availability of phenolic hydrogens as well as the potential for stabilization of the resulting phenoxyl radicals formed by hydrogen donation (Prochazkova et al., 2011). For example, Lee et al. (2010) showed that the DPPH radical scavenging activity for the flesh part of
>
Hydroxyl radical scavenging activity
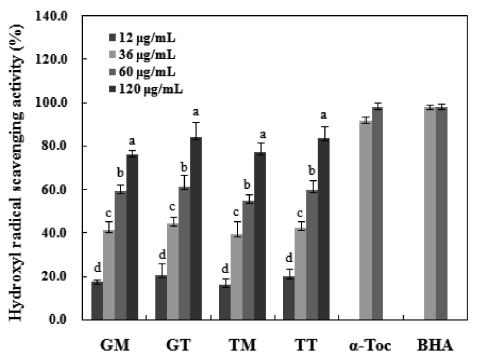
Among reactive oxygen species, hydroxyl radicals are the most reactive and often induce severe oxidative damage to important biomolecules such as proteins, DNA, PUFA and nucleic acids, causing aging, cancer and other several diseases (Aruoma, 1998). Fig. 5 shows the hydroxyl radical scaveng-ing activities and positive standards values of the carotenoids isolated herein. These values were compared with those of α-tocopherol and BHA to assess the antioxidant capacities of the carotenoids at concentrations of 12 to 120 μg/mL. Samples harvested in March exhibited the strongest radical scavenging
activities. GT and TT showed maximum scavenging values of 84.4% and 83.9%, respectively, at a concentration of 120 μg/mL. Similarly, the GM and TM carotenoids also increased in a dose-dependent manner. Conversely, the positive con-trols (α-tocopherol and BHA) did not show dose dependency and exhibited hydroxyl radical scavenging activities of ap-proximately 98.0% and 98.3%, respectively. Some seaweed extracts show weak hydroxyl radical scavenging activities of
approximately 40% at mg levels (Meir, 1995; Siriwardhana et al., 2003). However, the 90% MeOH fraction evaluated in a study of
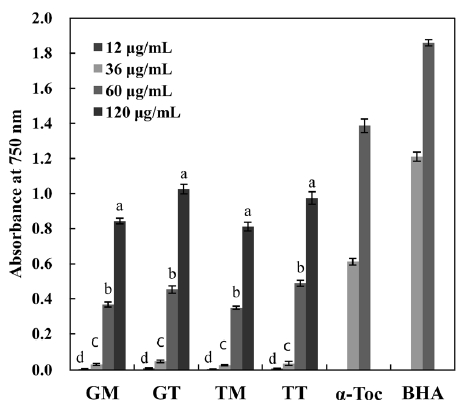
The concentration dependency of antioxidant activity was investigated as a function of reducing power (Fig. 6), as this gives a general view of reductones present in the sample. The reducing power increased with increasing concentration in all samples. Ganesan et al. (2008) reported that the reducing power of MeOH extracts from some red seaweeds were low at the mg level, as indicated by an optical density (OD) of <0.2. However, in the present study, the carotenoids of
In summary, both tunic and muscle yielded high levels of carotenoids, with values that are comparatively higher than those seen in other seafood species. The average values in the GM, GT, TM and TT samples were 49.1 mg/100g, 56.7mg/100g, 42.0 mg/100g and 50.2 mg/100g, respectively. The crude carotenoids of both tunic and muscle samples were tested for antioxidant activities, and both exhibited weak DPPH scavenging activities. However, these samples exhib-ited a strong inhibitory effect against linoleic acid peroxida-tion, with the degrees of inhibition being comparable to those of the α-tocopherol and BHA standards. Furthermore, all samples showed strong hydroxyl radical scavenging activi-ties and reducing power, particularly the GT and TT samples collected in March, which exhibited values similar to those of α-tocopherol.