Functional foods may contain bioactive peptides since they exert a physiological effect in the body. These peptides are released from dietary protein by enzymatic hydrolysis or dur-ing processing. Bioactive peptides that regulate blood pres-sure are believed to be inhibitors of angiotensin-Ι-converting enzyme (ACE). Those peptides are, in general, inactive within the sequence of the parent protein, but can be released dur-ing gastrointestinal digestion or food processing (Shahidi and Zhong, 2008). Recently, many ACE-inhibitory peptides have been isolated and characterized from various protein hydroly-sates such as cheese (Smacchi and Gobbetti, 1998), milk pro-tein (Gobbetti et al., 2000), egg white (Miguel et al., 2007), plant protein (Dziuba et al., 1999; Wu et al., 2008), meat (Jang and Lee, 2005), and marine resources (Je et al., 2005; He et al., 2006). Various ACE-inhibitory peptides have also been isolated from fish protein,
ACE (EC 3.4.15.1) is a circulating enzyme that partici-pates in the rennin-angiotensin system and plays an important physiological role in regulating blood pressure. ACE acts as an exopeptidase that cleaves dipeptides from the C-terminus of various oligopeptides. It converts the inactive form of de-capeptide (angiotensin Ι) to a potent octapeptide vasoconstric-tor (angiotensin II), and inactivates the catalytic function of bradykinin, which has a depressor action (Ondetii et al., 1977; FitzGerald et al., 2004).
A giant jellyfish,
Papain hydrolysis of the jellyfish was conducted under the following conditions. First, jellyfish samples were lyophilized to remove excess water. Enzymatic hydrolysis (100 mL) was carried out for 4 h with 10% (w/v) lyophilized jellyfish at 60℃ and pH 6.0. The ratio of enzyme to reaction volume was varied from 0 to 2% (w/v). Each enzyme reaction was stopped by heat treatment at 90℃ for 15 min. The resultant slurry was centrifuged at 3,000 g for 10 min, and the supernatant was ly-ophilized and then used for analysis. The degree of hydrolysis was defined as the proportion (%) of α-amino nitrogen with respect to the total-N in the samples (Taylor, 1957).
Degree of hydrolysis (%) = (h/htot) × 100,
where htot is the total-N of lyophilized samples, and h is the quantity of α-amino nitrogen.
>
Determination of ACE-inhibitory activity
ACE-inhibitory activity was determined using the modified method of Cushman and Cheung (1971). The standard reac-tion mixture contained 5 mM Hip-His-Leu as a substrate, 0.3 M NaCl, and 5 mU ACE in 50 mM sodium borate buffer (pH 8.3). A sample (50 μL) was added to enzyme solution (50 μL) and then mixed with 8.3 mM Hip-His-Leu (150 μL) contain-ing 0.5 M NaCl to obtain the same concentration as the stan-dard reaction mixture. After incubation at 37℃ for 30 min, the reaction was stopped by the addition of 1.0 N HCl (250 μL). The resulting hippuric acid was extracted by addition of 1.5 mL ethyl acetate. After centrifugation (800 g, 15 min), 1 mL of the upper layer was transferred to a new glass tube and evapo-rated at room temperature for 2 h in a vacuum. The extracted hippuric acid was dissolved in 3.0 mL distilled water, and the absorbance at 228 nm was measured using a spectrophotom-eter (model U-3210; Hitachi Co., Tokyo, Japan).
>
Fractionation of jellyfish hydrolysates
The papain hydrolysate showing the greatest inhibitory ac-tivity was selected and the resultant hydrolysate was fraction-ated through an Amicon Millipore membrane (1 kDa cutoffs; Amicon, Beverly, MA, USA). The resultant fraction (FII) was lyophilized and then stored at -82℃ until required.
>
Purification of ACE-inhibitory peptide
The active fraction (FII) was further purified by Sephadex LH-20 (25 × 250 mm) gel filtration chromatography, eluting with 30% methanol at a flow rate of 0.5 mL/min, and reverse-phase HPLC eluting with a linear gradient of MeOH-H2O (A eluent; H2O:MeOH:TFA = 90:10:0.1 (v/v/v), B eluent; MeOH:H2O: TFA = 90:10:0.1 (v/v/v)) at a flow rate of 2 mL/min (C18 5 μM ODS 3100A, 10 × 250 mm, UV detection at 214 nm; Phenomenex). To obtain pure peptide, a Jupiter Pro-teo C12 column (90 A, 10 ㎛, 21.2 × 150 mm; Phenomenex) was used at a flow rate of 2 mL/min with a linear CH3CN-H2O gradient (A eluent; H2O:CH3CN:TFA = 95:5:0.1 (v/v/v), B eluent; CH3CN:H2O: TFA = 55:45:0.08 (v/v/v).
>
Structure of the purified peptide
The IR spectra were recorded on a Fourier transform (FT)-IR model IFS-88 spectrometer (Bruker, Billerica, MA, USA). NMR spectra were recorded on a JEOL JNM ECP-400 (400 MHz for 1H, 100 MHz for 13C) spectrometer (JEOL Ltd., Tokyo, Japan). Chemical shift (δ) values were expressed in ppm and were referenced to the residual solvent signals with resonances at δH/δC, 7.26/77.0 (CDCl3). To calculate molecu-lar weights, purified samples were processed on an 1100 LC/MSD spectrometer (Agilent Technologies, Santa Clara, CA, USA) with direct injection onto an electrospray interface in the positive or negative mode. The structure of the purified peptide was conformed using Chemdraw 11 (CambridgeSoft, Cambridge, MA, USA).
>
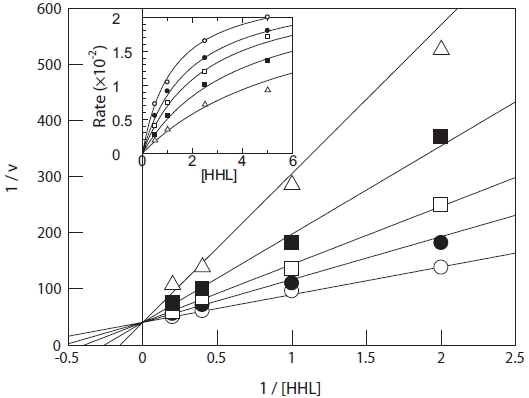
IC50 values and kinetic analysis
The IC50 value of the purified peptide was determined by a standard method, and was defined as the concentration of inhibitor required to inhibit 50% of ACE activity. For a Line-weaver-Burk plot, 0-4 μg/mL of purified peptide was added to the reaction solution as an inhibitor, and the inhibition pattern and
>
Papain digestion of jellyfish
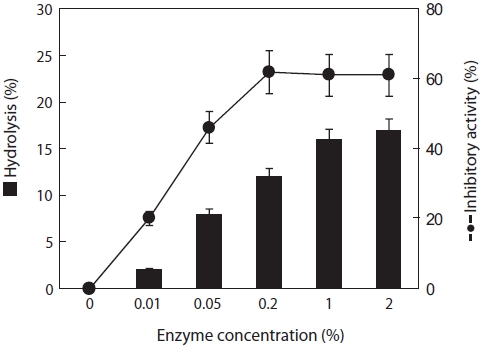
The hydrolysis rates of jellyfish mesogloea ranged from 2 to 17%, depending on the papain concentration used. Ex-amination of the ACE-inhibitory activity of each hydrolysate revealed that the activity increased significantly by as much as 62% after 4 h digestion, although negligible activity was detected in intact jellyfish. The greatest inhibitory activity was obtained with an enzyme concentration of 0.2% (w/v), and it then leveled off even in a prolonged reaction (Fig. 1). Pa-pain can digest most protein substrates and exhibits a broad substrate specificity, cleaving the peptide bonds of basic amino acids, leucine, or glycine. Because of this broad sub-strate specificity, many peptides and amino acids have been produced, leading to higher ACE-inhibitory activity. Lee et al. (2001) and Kim et al. (1996) reported that no relationship existed between the degree of hydrolysis of laver and fresh-water fish and ACE-inhibitory activity. In the case of laver hydrolysate, both neutrase and papain achieved a similar de-gree of hydrolysis (50.7% and 51.3%, respectively) and ACE-inhibitory activity (34.3% and 34.9%, respectively), whereas the hydrolysis rate and ACE-inhibitory activity of jellyfish differed markedly. This indicates that different substrates will show clear differences in reaction pattern.
>
Fractionation and characterization of papain hydrolysate
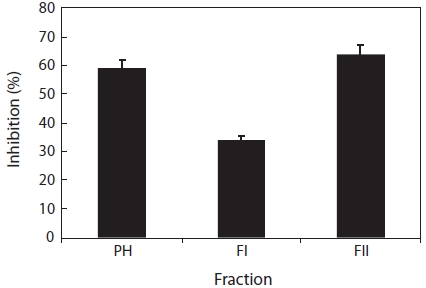
The papain hydrolysate was fractionated by ultrafiltration (1 kDa MWCO) and the activity determined. The resulting fraction (FII, <1 kDa) possessed 61.1% of the total activity, whereas the retained fraction (FI, >1 kDa) possessed 35.0% (Fig. 2). These findings suggested that the most active com-pounds were present in the FII fraction and that this activity was newly exposed by papain treatment because no recogniz-able inhibitory activity was detected before papain hydrolysis. Molecular weights (Mw) of fraction FII were less than 1 kDa, since a 1 kDa MWCO membrane was used.
To inhibit ACE
the molecular weight of hydrolysate and the specificity of the ACE-inhibitory activity, and revealed that activities increased with decreasing molecular weight. However, we were unable to detect a correlation between molecular wieght and ACE-inhibitory activity.
>
Purification of the ACE-inhibitory peptide
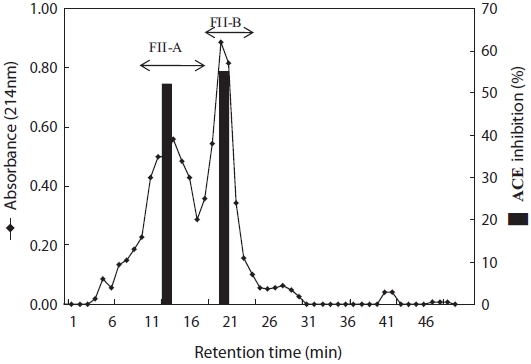
To purify the ACE-inhibitory peptide, fraction FII was sub-jected to a Sephadex LH-20 column, and two major fractions were obtained: FII-A and FII-B (Fig. 3). The ACE-inhibitory activities of FII-A and FII-B represented 54.01% and 55.08%, respectively. FII-B was further purified by HPLC using a re-verse-phase column (ODS C18). Inhibitory activity was detect-ed in a wide range of eluted fractions (FII-B(I)-FII-B(VII)), indicating that many inhibitory peptides were exposed by papain treatment. For further purification, FII-B(IV), which
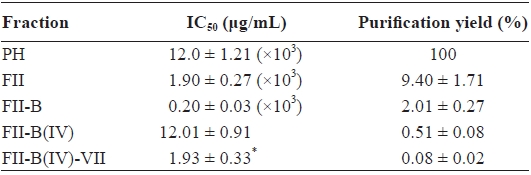
[Table 1.] ACE-inhibitory activity and purification yield of each step
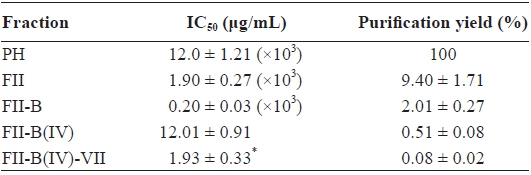
ACE-inhibitory activity and purification yield of each step
exhibited the highest ACE-inhibitory activity, was applied to another reverse-phase column (ODS C12), and a pure active peptide, FII-B(IV)-VII, was obtained (Fig. 4). The purifica-tion yield and IC50 value of FII-B(IV)-VII were 0.08 ± 0.02% and 1.93 ± 0.33 μg/mL, respectively (Table 1). Based on the molecular weight (294.16 Da) determined by an Agilent 1100 LC/MSD spectrometer, the IC50 value was 6.56 ± 1.12 μM.
Lee et al. (2005) purified ACE-inhibitory peptides from goat’s milk casein hydrolysates of pepsin, and showed their sequences to be Ala-Tyr-Phe-Tye and Pro-Tyr-Tyr. Gao et al. (2010) also produced ACE-inhibitory peptides from papain-digested cottonseed hydrolysate, and calculated the IC50 val-ues to range from 0.159 to 0.792 mg/mL, even though they did not elucidate the structure.
In this work, we hydrolyzed jellyfish with papain and puri-fied an ACE-inhibitory peptide (FII-B(IV)-VII) from the hy-drolysate. These findings led us to postulate that many proteo-lytic enzymes, including papain, may be applied to produce ACE-inhibitory peptides of varying molecular weight from several jellyfish species.
>
Structure of the purified peptide
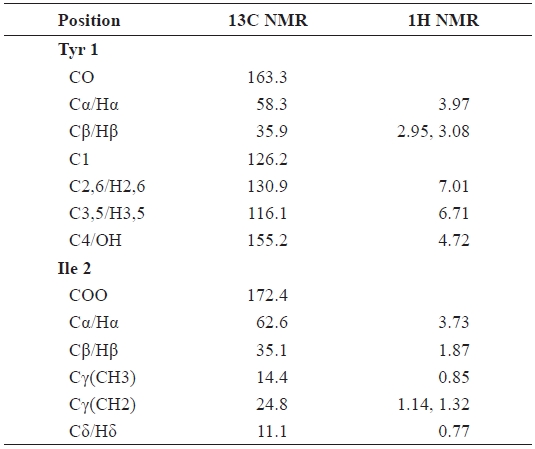
To elucidate the structure of FII-B(IV)-VII, several tech-niques were employed. The IR spectrum showed an NH/OH vibrational frequency at 3,398 cm-1 and peptide bond amine peak (CN) at 1,203 cm-1, whereas the carbonyl and broad peaks of the cyclic ring appeared at 1,672 and 1,468 cm-1, re-spectively. These IR data indicated that the peptide contains a phenolic ring.
The 1H, 13C NMR, and DEPT data allowed assignment of seven methine, two methylene, and two methyl groups. The remaining quaternary centers consisted of a carbonyl (172.4 ppm), hydroxyl (155.2 ppm), and double-bonded (163.3 ppm) oxygenated carbon signals. The 1H NMR spectrum showed that the peaks at δ 1.14, 1.32, 2.95, and 3.08 ppm were related to the methylene protons of the ester group (Table 2). These data convinced us that FII-B(IV)-VII contains tyrosine and isoleucine. The methine protons of isoleucine appeared at δ 1.87 and 3.73 ppm, and those of tyrosine appeared at δ 3.97 ppm. The methine protons attached to the cyclic side chain appeared at δ 6.71 and 7.01 ppm as a doublet. The 13C NMR spectrum showed two carbonyl peaks at δ 172.4 (C=O ester) and δ 163.3 ppm (C=O amide), δ 116.1-155.2 ppm (benzilic carbons), δ 35.1, 58.3, and 62.6 ppm (methine carbons), δ 11.1 and 14.4 ppm (methyl carbons), which correlated with the structure of tyrosine-isoleucine (Tyr-Ile) (Table 2). These data strongly suggest that the purified peptide, FII-B(IV)-VII, is a Tyr-Ile dipeptide, which is a novel dipeptide reported for the first time in this work. The molecular weight and isoelec-tric point (pI) of Tyr-Ile were calculated to be 294.35 Da and 5.9, respectively, using the ChemBioDraw 11 program, cor-responding to the LC/MS data (294.16 Da).
Kawasaki et al. (2000) reported that a dipeptide (Val-Tyr)
[Table 2.] 1H and 13C NMR chemical shifts for the FII-B(IV)-VII
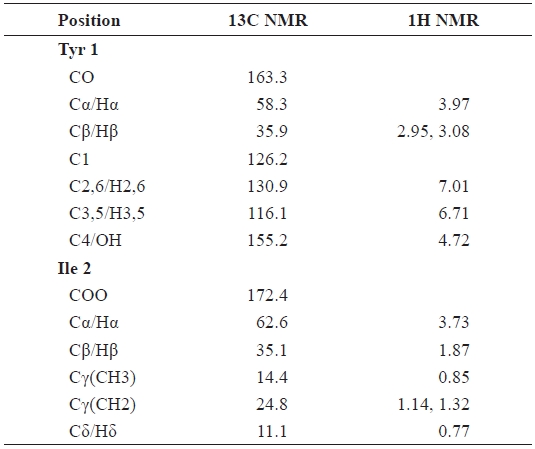
1H and 13C NMR chemical shifts for the FII-B(IV)-VII
purified from sardine muscle hydrolysate had a significant an-tihypertensive effect on mildly hypertensive subjects via ACE inhibition. Erdmann et al. (2006) reported that sardine muscle hydrolysate contained a Met-Tyr dipeptide that showed ACE-inhibitory activity and was capable of diminishing free radical formation in human endothelial cells.
>
IC50 value and kinetic analysis
The ACE inhibition pattern of the purified peptide was in-vestigated by applying a Lineweaver-Burk plot. Tyr-Ile acts as a competitive inhibitor of ACE, suggesting that Tyr-Ile from
Some antihypertensive drugs are known to produce side ef-fects, such as an abnormal elevation of blood pressure. How-ever, jellyfish is a favorite seafood in Southeast and East Asian nations. Thus, jellyfish may be a useful functional food for maintenance of blood pressure within the normal range. Our results also suggest that an ACE inhibitor derived from
![Separation chromatogram of the FII-B(IV) by using C12 reverse-phase column. The resultant active fraction (FII-B(IV)) after C18 column chromatography was loaded on a Jupiter Proteo C12 column (90A 10 ㎛ 21.2 × 150 mm) and eluted with a linear gradient of CH3CN-H2O [A eluent; H2O:CH3CN:TFA = 95:5:0.1 (v/v/v) B eluent; CH3CN:H2O:TFA = 55:45:0.08(v/v/v)] at a flow rate of 2 mL/min. The inhibitory activity of each elute from I to X was determined and the resultant active fraction FII-B(IV)-VII was obtained.](http://oak.go.kr/repository/journal/11592/E1HKAL_2011_v14n4_283_f004.jpg)