The phylum rotifer is distributed worldwide, and more than 2,000 species have been described (Dumont, 1983) from freshwater, brackish water, and seawater areas. Although most have originated from inland freshwater ecosystems, several rotifers have been reported from marine and/or brackish water zones. For instance, about 20 of the 32 species comprising the genus
Dumont (1983) reported that the
Two common live-food-organism rotifers,
The area from which rotifers were collected is located in the Hwajinpo coastal lagoon, Gangwon Province, South Korea. The Hwajinpo coastal lagoon is located at the northern tip of the east coast of South Ko-rea. The lagoon is strictly un-der military control and is therefore a military protected area that cannot be contaminated by
>
Isolation and stock cultures for mono specific conditions
The samples taken from Hwajinpo lagoon were shipped to our laboratory. Then, isolated rotifers were acclimated from low salinity (1-19 psu) to natural seawater salinity (32 psu). Isolation and cultures were established based on the rotifer mono-species culture method (Jung et al., 1997) and the ma-rine rotifer axenic culture method (Jung et al., 1998; Jung and Hagiwara, 2001). Stock cultures of isolated Hwajinpo rotifers were grown in filtered natural seawater at 32-psu salinity and a water temperature of 25℃ and were fed on centrifuged mi-croalga,
>
Morphological description and identification
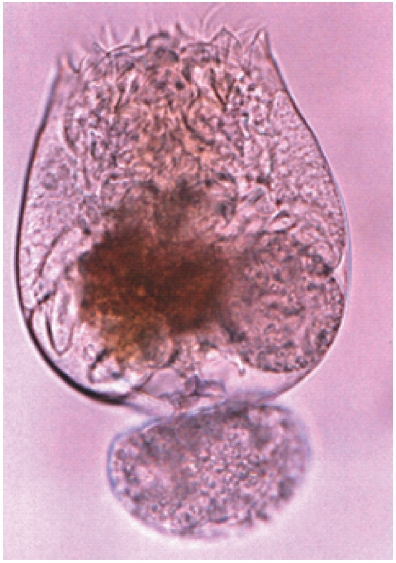
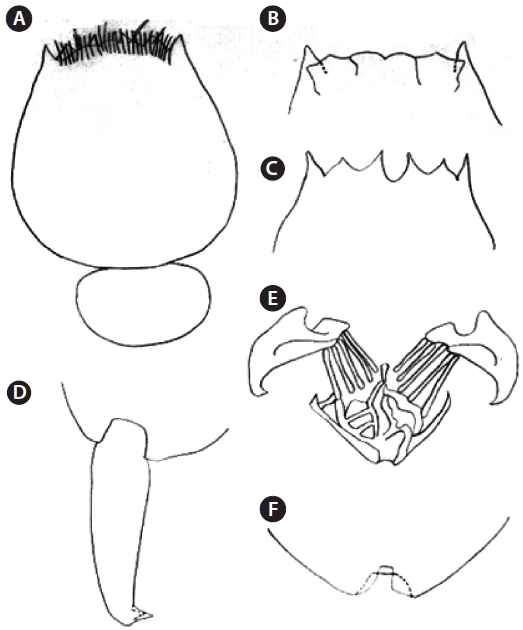
After harvesting from an indoor culture tank, the isolated Hwajinpo rotifer strain was fixed in 5% buffered formalin for taxonomical identification. A specimen was dissected by light microscopes (Olympus CH-2 and SZH, Japan), and the mor-phological characteristics of this rotifer strain were described. Identification of the new isolated rotifer was performed fol-lowing to Sudzuki’s (1964, 1996), and Wallace and Snell’s (1991) methods.
>
Measurement of abiotic environmental factors in Hwajinpo lagoon
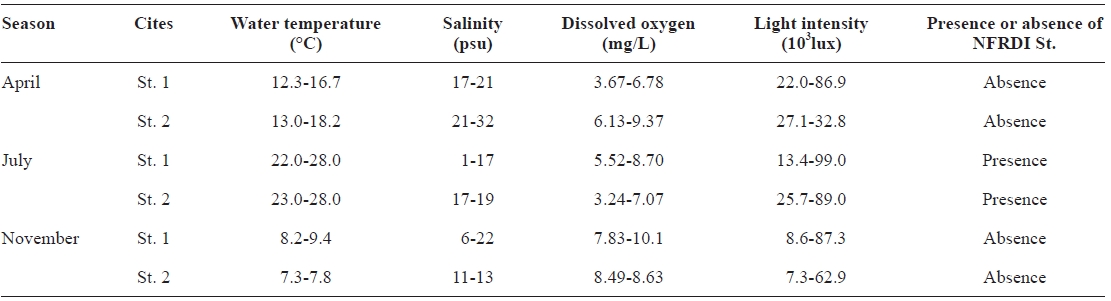
The water quality of Hwajinpo lagoon was investigated by measuring the most important abiotic environmental factors such as water temperature, salinity, dissolved oxygen, and light intensity using a YSI environmental monitoring system and a light meter (Digital instruments LM series). The mea-surements of these abiotic factors were conducted on three oc-casions (in April, July, and November of 2002).
>
Measurement of biological minimum size
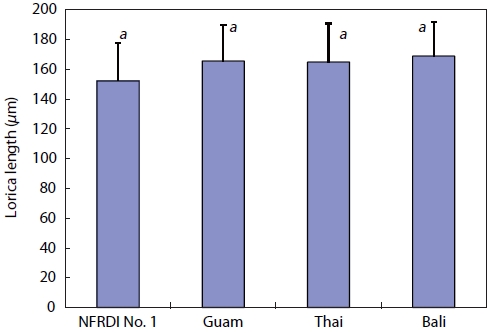
The biological minimum size of isolated Hwajinpo rotifers was measured and compared with that of three other
A
>
Isolation and morphological description of the Korean Hwajinpo lagoon rotifer strain
A new brackish rotifer strain was isolated from the uncon-taminated Korean Hwajinpo lagoon and classified as
>
Measurement of Hwajinpo lagoon environmental factors
The temperatures observed during the three sampling months (April, July, and November of 2002) ranged from 7.3 to 28.0℃. The highest water temperature (28.0℃) was observed in July (during summer), with lower temperatures in April (12.3-18.2℃) and November (7.3-9.4℃) (Table 1). The new NFRDI N° 1 rotifer strain was only collected in July (summer) and was not found at low water temperature (April: 12.3-18.2℃ and November: 7.3-9.4℃). The annual water temperature of Hwajinpo lagoon reached a minimum in win-ter of 3.6℃ and a maximum in summer of 28.0℃ (Table 1).
The salinity of Hwajinpo lagoon differed with measure-ment site and period. Salinity ranged from 1 to 32 psu, with lower salinity at sites more distant from the sea (St. 1 in Table 1) and higher salinity at sites near the sea (St. 2 in Table 1). Additionally, high salinity was found in April, whereas inter-mediate and low salinities were observed in July and Novem-ber, respectively. The NFRDI N° 1 rotifer was found in July where salinity ranged from 1 to 19 psu (Table 1).
The variation in the amplitude of dissolved oxygen concen-trations and light intensities was very great. Dissolved oxygen concentrations ranged from 3.24 to 10.1 mg/L, and light inten-sities ranged from 7,300 to over 99,000 lux (Table 1).
>
Measurement of biological minimum size
The biological minimum size of the NFRDI N° 1 rotifer strain was very small compared with those of the rotifer strains common to Guam, Thai, and Bali. The average lorica length of the NFRDI N° 1 strain was 152.23 ± 25.58 ㎛ (mean ± SD,
[Table 1.] Investigation of water quality in Hwajinpo lagoon on the each different sampling month
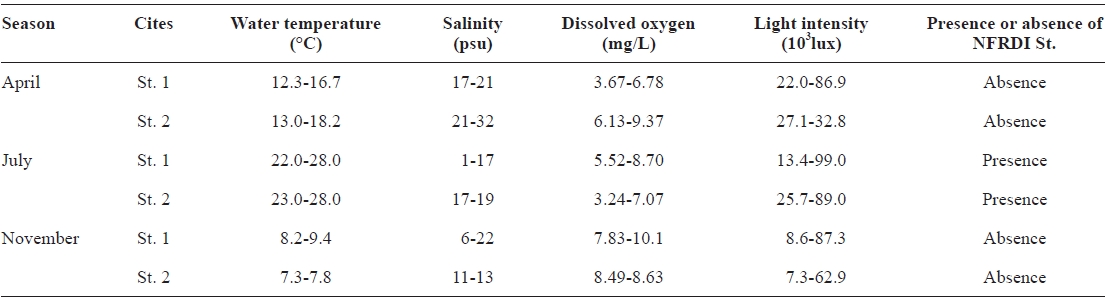
Investigation of water quality in Hwajinpo lagoon on the each different sampling month
The new Korean brackish
Salinity ranged from 1 to 32 psu. Lower salinity was ob-served at sites distant from the seacoast (St. 1 on Table 1), and higher salinity at sites close to the seacoast (St. 2 on Table 1). Additionally, high salinity was found in April, whereas inter-mediate and low salinities was observed in July and Novem-ber, respectively, indicating that the occurrence of the NFDRI N° 1 strain was not associated with salinity.
Most
Most of the monogonont rotifer life cycle occurs in the amictic phase, but under particular conditions, sexual repro-duction occurs within rotifer populations when initiated by a specific environmental stimulus (
All live-food rotifers used at fish larval rearing stations originated from eel culture tanks or from the mangrove zones of subtropical and tropical regions and therefore cannot be collected from the wild and used as valuable live-food roti-fers. However, the present study documented the existence at Hwajinpo lagoon, Gangwon Province, South Korea of a new rotifer named the