Aluminum (Al) is a nonessential biological element that is toxic to fish, interfering with ion regulation (Verbost et al., 1989; Booth et al., 1988) and respiratory function (Karlsson-Norrgren et al., 1985; Wood et al., 1988) in the gills. Fish re-production is also impaired by this metal (Haux et al., 1988; Rask et al., 1990). For example, the spawning of perch is de-layed in acidic water with high Al levels (Rask et al., 1990). Such environments have been shown to impair oogenesis due to the poor accumulation of egg yolk in the perch
After absorption by fish, Al accumulates in the liver (Buer-gel and Soltero, 1983; Norrgren et al., 1991; Exley, 1996), where vitellogenin (VTG, a calcium-binding phosphlipogly-co-protein), an egg yolk precursor protein, is synthesized in mature females under estrogen (estradiol-17β, E2) stimulation. VTG is also used as a nutrient source for embryonic and larval development until the beginning of feeding.
In recent years, attention has been paid to the potential significant impacts of ocean acidification on many calcifying organisms, especially corals and other invertebrates that pre-cipitate aragonite skeletons (Hoegh-Guldberg, 2009). In con-trast, the range of impacts that ocean acidification may have on marine fish remains poorly understood. Acidic water, in-cluding high Al concentrations may damage the reproductive physiology of stationary fish in coastal zones near estuarine outflow. However, few studies have investigated the effects of Al on the reproductive physiological processes of marine fish species. We used immature rockfish,
Immature
Fish were intraperitoneally injected with E2 (5 mg/kg BW) in 70% ethanol and Al (AlCl3·6H20, Wako) (0.1, 1, 5, and 10 mg/kg BW) in distilled water twice at 3-day intervals, and blood samples were obtained from the fish (
Fish were anesthetized with 2-phenoxyethanol and their blood was collected by injecting the tail of the fish and by draining the blood into heparinized capillary tubes. Plasma was separated by centrifugation (350 g, 8 min) and frozen at -20℃ until the analysis of VTG, ALPP, Ca, and GPT concen-trations.
>
Effect of Al on VTG synthesis
Protein concentrations of the plasma were determined ac-cording to the method of Bradford (1976). Plasma proteins were also analyzed by 5-20% sodium dodecyl sulfate poly-acrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli (1970). After SDS-PAGE, the integrated optical density (IOD) of the VTG band was measured using a Bio Image System (Millipore, Bedford, MA, USA) and was expressed as a percentage of the IOD of the total proteins in-cluding VTG. Minor subunits of VTG were not considered VTG, because the subunits constituted only a fairly small part of VTG and overlapped with other proteins (Kwon et al., 1993).
Fish were administered with Al twice at 3-day intervals, and were sampled 7 days after the last administration. The dosage was the same as described above. Plasma was separated from the blood of the fish and analyzed for the main VTG band by SDS-PAGE.
>
Effect of Al on ALPP and Ca concentrations
ALPP was analyzed from a 0.01 ml sample according to the method of Wallace and Jared (1968) and determined by colo-rimetric assay of the acidified phosphomolybdate complex using a commercially available kit (670-A; Sigma, St. Louis, MO, USA). This kit consists of the necessary reagents includ-ing a phosphorus reagent and calcium/phosphorus standard. The absorbance of the standards and samples were measured by an atomic absorption spectrophotometer (10-AU Fluoro meter; Turner Designs, Sunnyvale, CA, USA) at 340 nm.
Ca concentration was examined using a Ca assay kit (Asan Pharm. Co., Ltd, Seoul, Korea) by the
>
Effect of Al on GPT concentration
GPT was examined using an assay kit (Asan Pharm. Co., Ltd) flowing the method of Reitman-Frankel (Racicot et al., 1975). This kit consists of the necessary reagents including standard solution, reaction solution, assay solution, and 4 N NaOH solution.
The assay was conducted according to the kit instruction. Briefly, 1 mL reaction solution was added to 0.2 mL plasma in Al-administrated rockfish and then 0.4 N NaOH was added and mixed at room temperature for 10 min. Distilled water was used as the blank. The standard solution of GPT and sam-ples were measured by an atomic absorption spectrophotom-eter (10-AU Fluoro meter; Turner Designs) at 505 nm.
The body and liver weights of Al-administrated fish were examined, and HSI was expressed as liver weight × 100/ BW.
Fish were administered with Al twice at 3-day intervals and were dissected in the laboratory 7 days after the last adminis-tration. Liver samples were stored in a deep-freezer at -20℃
until analysis.
Liver samples were dried for 12 h at 80℃ and 0.1 g dried tissue was digested with 1.5 ml concentrated analytical HNO3 in a Teflon polytetrafluoroethylene tube in a microwave oven (Yasunaga et al., 2000; Anan et al., 2001). Al content was de-termined using an ICP-MS (HP-4500; Hewlett-Packard, Illi-nois, USA).
Data were analyzed by one-way analysis of variance (ANOVA) (Fisher protected least significant difference test). Fisher’s tests were also used to examine the significance of correlation coefficients. Significance was accepted at
>
Effect of Al on VTG synthesis
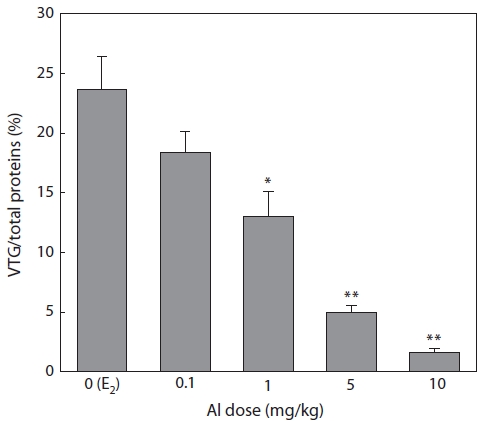
Plasma samples were extracted and then analyzed by SDS-PAGE in 7 days after E2 and/or Al administration. VTG ac-counted for 23.6% of the total proteins in the control group, but this value decreased with increasing Al dosage (Fig. 1). Significant inhibition was confirmed at Al dosages of 1, 5, and 10 mg/kg, under which VTG productions were reduced to 54.2% (
Immature rockfishes were intraperitoneally administrated with Al to compare the eventual dosage-dependent response and to avoid large amounts of Al-polluted waste water dur-ing experiment periods. Enzyme linked immunosorbent assay is a sensitive method for VTG determination and has been widely used for this purpose. In the present study, however, SDS-PAGE was used to separate VTG before optical quan-tification. VTG production in the presence and absence of Al was expressed as a percentage of total proteins. This type of expression has the benefit of excluding the effects of varia-tion in the amount of protein applied to the gel. It also has the advantage of checking the effects of Al on the production of proteins other than VTG. A significant decrease in percentage indicates that the synthesis of VTG is more susceptible to Al than are other hepatocyte-derived proteins.
Al is a nonessential biological element and therefore fish may have no regulatory mechanisms for it. The chemical form of Al depends on pH. At neutral pH, it occurs as Al(OH)3 precipitate and/or in a soluble Al(OH)4- form (Verbost et al., 1992). As no precipitates were found, Al exerted its effect on vitellogenesis as an ion of Al(OH)4-. Al is toxic to fish, inter-fering with ion regulation (Booth et al., 1988) and respiratory function (Wood et al., 1988) in the gills. Fish reproduction may also be impaired by this metal (Rask et al., 1990). Hwang et al. (2000) reported that Al inhibits VTG and its mRNA in-duction by E2 in the primary culture of hepatocytes in the rain-bow trout Oncorhynchus mykiss. Nakao et al. (2002) reported that Al inhibits E2-induced VTG production in immature and male salmon
>
Effect of Al on ALPP concentration
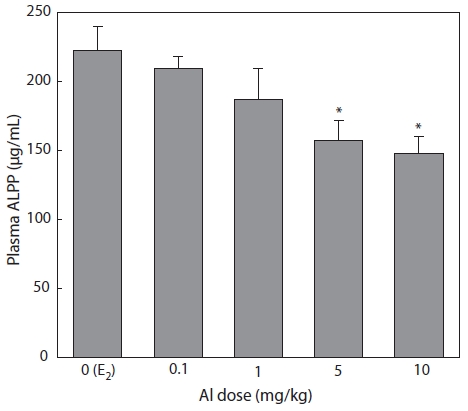
Plasma samples were extracted and analyzed by colori-metric assay 7 days after E2 and/or Al administration. Plasma ALPP decreased in a concentration-dependent manner in all groups of Al-administered fish compared to the control group that had been treated with E2 only. Significant inhibition was confirmed at Al dosages of 5 and 10 mg/kg, at which ALPP concentrations were reduced to 70.7% (
Emmersen et al. (1979) reported that plasma ALPP concen-tration increased in male flounder
>
Effect of Al on Ca concentration
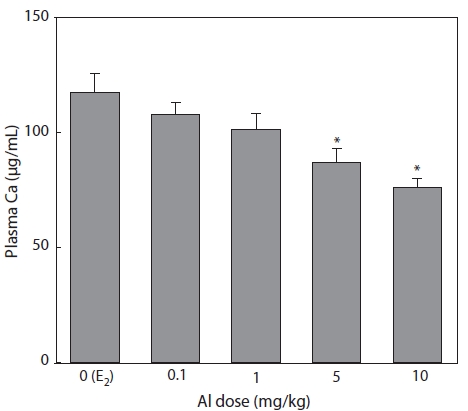
Plasma samples were extracted and analyzed by the
Nagler et al. (1987) and Tinsley (1985) reported that the concentration of plasma Ca can also indicate circulating VTG.
Ca binds to VTG, so that increased Ca may be mainly due to an increase in the protein-bound calcium fraction of the blood (Bjorsson and Haux, 1985). In the present study, the reduc-tions in Ca concentration induced by Al administration were similar to those of VTG. In the flounder
>
Effect of Al on GPT concentration
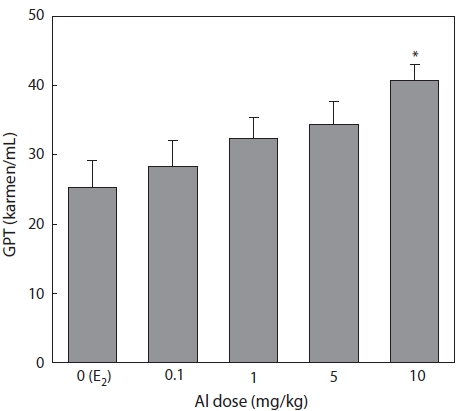
Plasma samples were extracted and analyzed following the methods of Reitman-Frankel 7 days after E2 and/or Al administration. GPT increased in a concentration-dependent manner in all groups of Al-administered fish compared to the control group treated with E2 only. A significant increase was confirmed at an Al dosage of 10 mg/kg, at which GPT was 25.3% higher than that of the control (Fig. 4).
The concentration of the plasma enzyme GPT has fre-quently been used to detect eventual damage to the liver cells. Hwang and Kang (2002) reported VTG induction by exog-enous E2 damage to hepatocytes, and the concentration of plasma GPT was temporarily increased in immature
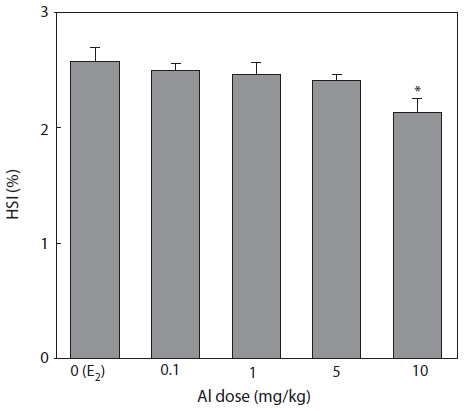
HSI was measured 7 days after E2 and/or Al administration (Fig. 5). HSI decreased in a concentration-dependent manner in all groups of Al-administered fish compared to the control group administered with E2 only. A significant reduction was confirmed at an Al dosage of 10 mg/kg, at which HSI was reduced to 85.3% of the control (Fig. 5).
Emmersen et al. (1979) reported that HSI increases in fish with E2, because the fish liver is primarily hypertrophic for VTG synthesis. Hwang et al. (2007) also reported increases in HSI by 4-nonylphenol administration similar to those induced by E2. In the present study, Al inhibited E2-induced primary hypertrophy in the liver which reduced HSI.
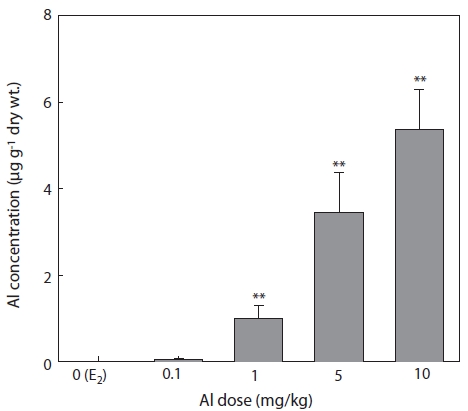
The concentrations of Al in the liver 7 days after E2 and/or Al administration are shown in Fig. 6. Al concentration increased in a concentration-dependent manner in all groups of Al-administered fish compared to the control group treated with E2 only (Fig. 6). Significant increases were confirmed at Al dosages of 1, 5, and 10 mg/kg, at which Al concentrations were 1 ± 0.29, 3.46 ± 0.91, and 5.38 ± 0.92 μg/g dry wt, re-spectively (Fig. 6).
The livers of vertebrates such as fishes are an important organs for storing metals and for detoxification by biotrans-formation (Brown et al., 1984; Olsson et al., 1989). Due to its higher exposure to metal concentration compared to other tis-sues, the liver can also be used for the bio-monitoring of coast-al pollution (Von Landwust et al., 1996; Broeg et al., 1999).
In the present study, as Al levels increased, so did GPT levels, whereas a decreasing HSI indicated that Al affected
the physiological activity of the liver. The internal accumula-tion of metals in aquatic organisms such as fishes is attributed to the introduction of dissolved metals through their diet or through the gills as they breathe.
Although the results of this study are inconsistent with the natural absorption of metals, they are very helpful in that the eventual dose-dependent responses could be induced and large amounts of Al-polluted wastewater could be avoided during the experiments, as described above. The accumulation of heavy metal in the liver tissue of fishes depends on temper-ature, age, aquatic chemicals, exposure time, and the amount of exposure (Pagenkopf, 1983; Heath, 1987; Goyer, 1991). De la Torre et al. (2000) reported that as
In conclusion, our results suggest that the concentrations of plasma ALPP and Ca could be utilized as an index of re-productive physiology in fish, because the changes in these concentrations after Al administration were similar to those of VTG. GPT concentration and HSI changed temporarily as in-creasing Al accmulation damaged hepatocytes. These results indicate that Al affects E2-induced vitellogenesis in immature rockfish