Korean rose bitterling
In addition, Korean rose bitterling has a small size, special life habits, and beautiful body color. As such, Korean rose bit-terling has generated much interest in researchers. For this, de-tailed information on their biology, especially early life histo-ry and reproductive cycle, effects of environmental factors on reproductive cycle, elongation of the ovipositor, nuptial color component, and effects of diet on nuptial color, have begun to be explored (Ahn, 1995; Kang et al., 2005). Recently, this spe-cies has also been considered as a candidate model for Korean research project of National Fisheries Research and Develop-ment Institute to address the risks associated with aquatic liv-ing modified organisms. Therefore, the producing of sterile triploid Korean rose bitterling stocks is needed for researching DNA transformation operation of Korean rose bitterling.
Triploid Korean rose bitterling have been produced with the purpose of preventing exotic strains from contaminating the local gene pool by unwanted reproduction in natural waters (Kim et al., 1994). More importantly, triploidy will have an important role to play in the regulation of genetically modified strains of Korean rose bitterling, with regard to recent applica-tions of recombinant DNA technology to this species (Nam et al., 1999). Also, tetraploid can providing a convenient way to Koproduce large numbers of sterile triploid fish through simple interploidy crosses between tetraploids and diploids (Guo et al., 1996). For these reasons, the development of a practical and convenient method to produce sterile triploid stocks and tetraploid stocks is needed.
The production of polyploid fish (
Effective, controlled releasing of the second ootid as well as the first cleavage is dependent on the type, intensity, and du-ration time of treatment (Thorgaard et al., 1981; Onozato and Yamaha, 1983; Thorgaard and Allen, 1987). Thus, control of first cleavage, a means of chromosome engineering, can affect enhancement of aquaculture over the short-term (Thorgaard, 1983). To produce sterile organisms, it can be applied to the induction of triploid. Further, control of first cleavage can be applied to the induction of tetraploidy, mitotic gynogenetic diploidy, and androgenetic diploidy using chromosome engi-neering. Therefore, to practice effective control of first cleav-age, an understanding of the temperature-dependent control of first cleavage is essential (Thorgaard, 1983; Mair, 1993).
In this study, we determined temperature-related cleavage rates or mitotic intervals, measured as the “Dettlaff unit” (τ0) for winter flounder, in order to establish efficient procedures for chromosome manipulation. The Dettlaff unit is the dura-tion in minutes of one mitotic cycle during early synchronous embryonic cleavage, or the interval between two consecutive cell divisions (Saat and Veersalu, 1996a; Shelton et al., 1997). When measured over a range of temperatures, the relationship of τ0 to temperature as determined by regression analysis can be used to predict developmental events that are influenced by temperature within a single species and between species with similar spawning biology (Dettlaff, 1986). To date, mitotic in-tervals (τ0) have been used to estimate the optimal times for chromosome manipulation in a variety of species such as the paddlefish
On June 2 2011, Korean rose bitterling
On June 8, 2011, eggs and sperms of Korean rose bitterling were collected from 30 females and 30 males; 40 eggs col-lected from 2 females and sperms collected from 2 males were artificially fertilized by a wet process. In a triplicate experi-ment, 200 fertilized eggs were collected from 10 females and 10 males.
To assess the temperature-dependency of the first cleavage and mitotic interval (τ0), the water temperatures were main-tained using temperature-controlled water baths set at 12, 16, 20, and 24℃. Samples were generally taken at 15 min inter-vals and fixed with 5% neutral formalin solution (50 mL of formalin, 3.25 g Na2HPO4·12H2O, 2.25 g KH2PO4, 950 mL DW) at 4℃ before observation. We measured the diameter of 10 fertilized eggs at 50× magnification under an optical micro-scope (Axiostar plus; Zeiss, Germany ) and microscope cam-era (Axiocam MR, Zeiss, Germany ). This experiment was performed in triplicate.
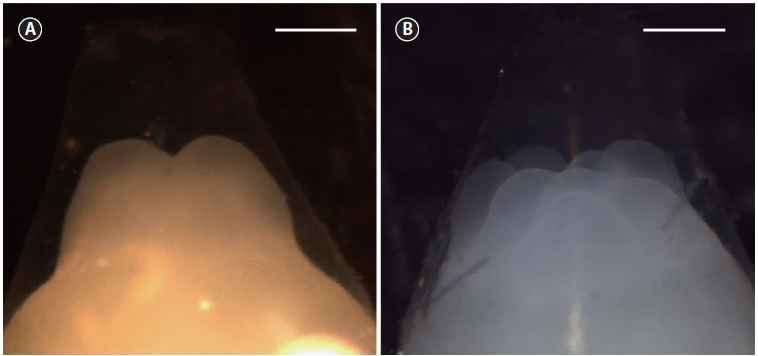
The time of appearance of the first cleavage furrow was recorded and used as the starting point for the timing of sub-sequent cell divisions. The times at which approximately 10% of the developing embryos reached the two- (τI) (Fig. 1A) and eight-cell (τIII) (Fig. 1B) stages were recorded. A value of 10% was selected according to the recommendation of Ignat’eva (1975). Mean mitotic cycle intervals (τ0) were calculated as τ0 = (τIII―τI)/2. The relationship between the mean mitotic in-terval and water temperature was examined by simple linear regression.
The mean diameter of the Korean rose bitterling
rean rose bitterling eggs do not contain oil globules (Park and Park, 1986).
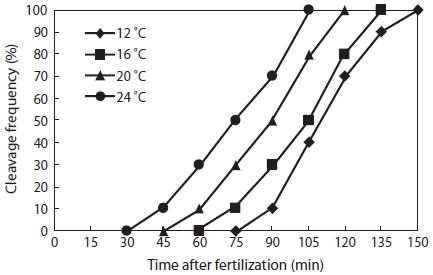
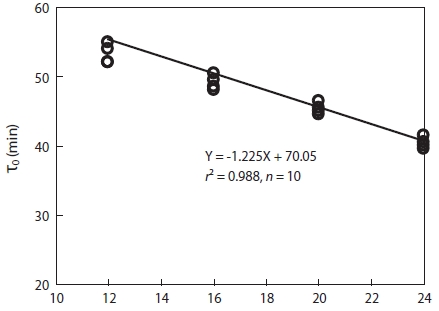
As shown in Fig. 2, the eggs of Korean rose bitterling showed faster development at higher temperature. The times at which eggs reached the one-cell stage at 12℃, 16℃, 20℃, and 24℃ were 150 min, 135 min, 120 min, and 100 min, re-spectively. As the water temperature was increased, the slope of the first cleavage frequency with elapsed time after fertil-ization increased, and approximately 30% of fertilized eggs reached first cleavage frequency every 15 min. As shown in Fig. 3, the mitotic intervals at 12℃, 16℃, 20℃, and 24℃ were 52 min, 48 min, 44.5 min, and 40 min, respectively, and there were strong negative correlations between mitotic intervals (τ0) and water temperature at all temperatures (Y = -1.225X + 70.05,
In an attempt to determine temperature-related cleavage rates and τ0, we found that Korean rose bitterling eggs under-went cleavage within the temperature range of 12 to 24℃. Based on our results, we determined that Korean rose bitter-ling eggs showed faster development and decreased mitotic intervals with increasing water temperature, which indicates strong negative correlations between τ0 and water tempera-ture. Although mitotic intervals and hatching time after fertil-ization were different, the trend of temperature-dependent τ0 in Korean rose bitterling is similar to those in black plaice
In addition, the relationships between mitotic interval and water temperature in fish were typically curvilinear, indicating that temperatures were within the range in which the species
of fish naturally spawn and develop (Saat and Veersalu, 1996a, 1996b; Park and Im, 2001; Park and Johnson, 2002; Park et al., 2006; Park and Im, 2010). The linear response of the τ0 against temperature attained in this study is in accordance with a study on the developmental rate for black carp, win-ter flounder, and black plaice (Shelton and Rothbard, 1993; Park and Johnson, 2002; Park and Im, 2010). However, addi-tional observations are needed. The available data suggest that the curves of the dependency of τ0 on temperature are highly species-specific. The species-specificity of the development rate can be used to identify the taxonomic ranges of different fish species. Considering the identity of the mitotic events and the short time intervals (τ0), the chromosome manipulations in Korean rose bitterling would be most efficient at temperatures between 16 and 20℃.
Therefore, this study demonstrated obvious specific and clear differences in the time of the first cleavage and mitotic intervals at different temperatures in Korean rose bitterling. Data obtained will be useful for the development of an op-timal treatment protocol for chromosome manipulation. Fur-ther, data on egg development will be valuable in the form of a biological aquaculture database. The results of this study and the investigations into seeding production by artificial fertil-ization, induction of triploid for producing sterile organisms, and induction of tetraploid, mitotic gynogenetic diploid, and androgenetic diploid in Korean rose bitterling will aid future research.