Lampreys are migratory, eel-shaped, jawless fishes. They have a multi-year stream-dwelling larval stage, metamor-phose into ichthyo-parasitic juveniles that feed for 1-2 years in marine or lentic habitats, and mature into migratory adults that spawn in streams (Hardisty and Potter, 1971). Lampreys are extant representatives of the ancient vertebrates of which about 40 species remain (Janvier and Lund, 1983). All the northern hemisphere lampreys belong to a single family, Pet-romyzontidae (Porter, 1980).
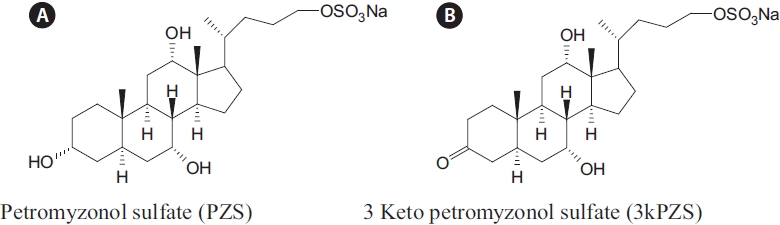
Since a bile acid, petromyzonol sulfate (PZS), as presented in Fig. 1A, chemically identified from sea lamprey four de-cades ago (Haslewood and Tokes, 1969), there has been ac-cumulating evidence that sea lamprey may use this bile acid as a chemical cue to guide the migrating lamprey to spawn-ing streams (Li et al., 1995; Li and Sorensen, 1997; Bjerselius et al., 2000; Polkinghorne et al., 2001). In addition, another bile acid, allocholic acid (ACA), was implicated as a second compound that might play an important role as a migratory pheromone (Bjerselius et al., 2000; Polkinghorne et al., 2001).
Interestingly, sexually mature male sea lamprey can pro-duce and release two bile acids, 3 keto petromyzonol sulfate (3kPZS), as presented in Fig. 1B, and 3 keto allocholic acid (3kACA), oxidized forms of the larval bile acids to attract conspecific for spawning (Li et al., 2002; Yun et al., 2003b). The concurrence of 3kPZS and 3kACA from mature lamprey, paralleled with the concurrence of PZS and ACA from larvae, led to speculation that sea lamprey have evolved a system to produce life stage specific chemical cues for their biological needs (Yun et al., 2003a).
It has been questioned whether other lamprey species use the same chemical cues for communication. The larval bile acid, PZS was found to exist in some larval lampreys, native to the Pacific coast, such as Pacific lamprey
extracts from the conditioned water with sexually mature male lampreys were analyzed with mass spectrometry (MS) and en-zyme linked immunosorbent assays (ELISAs).
Here I report that chestnut lamprey
Adult lampreys of the three species were collected from streams during their spring spawning migrations. Chestnut lamprey
Upstream migrating adult Pacific lamprey
River lamprey
Mature male and female American brook lamprey
>
Extraction of water conditioned with lampreys
The conditioned water samples from the four lamprey spe-cies were loaded onto Sep-Pak cartridges (Waters) where each Sep-Pak was eluted with 5 mL of methanol. The methanol elu-ates were pooled together, dried down, and subjected to MS and ELISA analyses.
Mass spectra were obtained using a JEOL HX-110 double-focusing fast atom bombardment (FAB) mass spectrometer (JEOL, Peabody, MA, USA), operable in either the positive or negative ion mode. Ions were produced by bombardment with a beam of Xe atoms (6 keV). The accelerating voltage was 10 kV and the resolution was set at 3,000. Samples were prepared for MS by drying down the high-performance liquid
chromatography fractions and re-dissolving them in methanol. High resolution MS was performed by peak matching with a resolution of 10,000. FAB MS was done at the NIH MS facil-ity at Michigan State University.
The extracts of water samples conditioned with lampreys were analyzed using the ELISAs for 3kPZS and PZS as de-scribed previously (Yun et al., 2002; Yun et al., 2003a). Serial dilutions of the extracts were assayed along with both 3kPZS and PZS standards in a range of 20 pg-10 ng/well, to confirm the identities of bile acid compounds in the samples. The en-zyme labels were generated by conjugating an enzyme, acetyl-choline esterase, to 3kPZ-24-HS and PZ-24-HS. Labels were diluted 1,000-2,000 times while the antibodies were diluted 200,000 times.
>
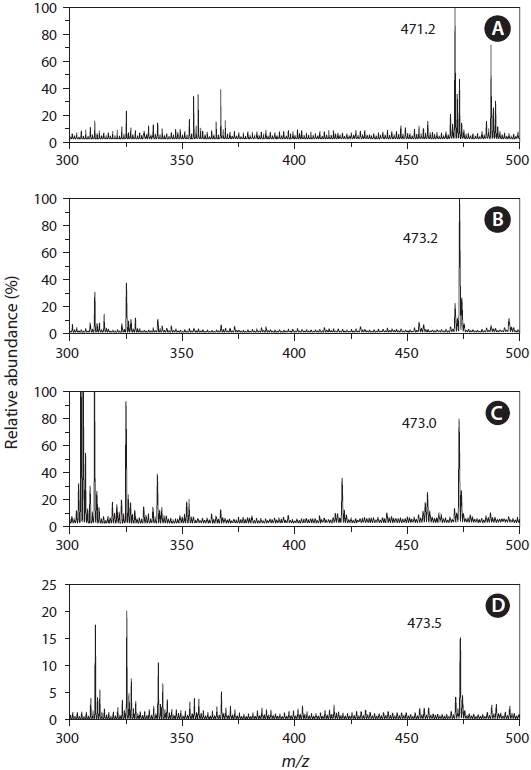
Identification of 3kPZS from silver lamprey by MS
MS analysis of the water extract from the male chestnut lamprey observed a major peak at
>
Identification of PZS from Pacific, river, and American brook lampreys by MS
MS analyses of the water extracts from both the Pacific, river, and American brook lampreys revealed the major peak at
>
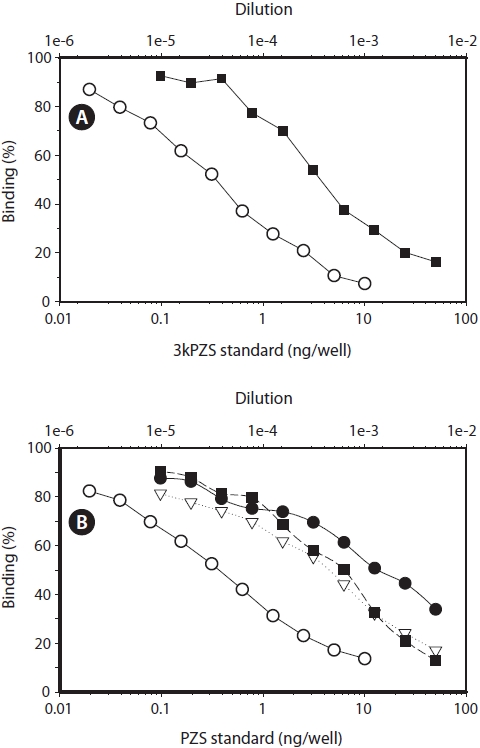
Further verification of the bile acids in the lam-preys (parallelism)
Close parallelism between the extract from the chestnut lamprey and 3kPZS standard was found (Fig. 3A). Similar close parallelism was also found between the extracts of the Pacific, river, and American brook lampreys and PZS standard (Fig. 3B).
It has been well established that sea lamprey have devel-oped an ability to produce life stage specific bile acid com-pounds to communicate between conspecifics (Li et al., 2003). I hypothesized that other lamprey species may have evolved the similar system because all the lamprey species have simi-lar life histories. However, the present study revealed that at least the chemical identities of bile acids released by all other lamprey species are not parallel with those of sea lamprey. Water samples conditioned with four different lamprey spe-cies identified only 3kPZS from chestnut lamprey, and only PZS from Pacific, river, and American brook lampreys. It can be speculated that the two
It is interesting to observe that sexually mature males of evolutionary ancestral species (chestnut and sea lampreys) can produce 3kPZS while the three recently derived species (Pacific, river, and American brook lampreys) can produce PZS. Although controversy exists about the phylogeny and
nomenclature of lamprey species (Bailey, 1980), phylogenies based on dentition (Hubbs and Potter, 1971; Porter 1980) have agreed that genus
Identification of life stage specific bile acids in sea lamprey led to a speculation that the enzyme 3α hydroxysteroid de-hydrogenase (3α HSD) may have intervened in conversion of 3kPZS to PZS (Yun et al., 2003b). The lack of 3kPZS in the
It makes ecological sense for some adult lampreys to re-lease forms of bile acids that are different from larval bile acids, as observed in ancient lamprey species such as chest-nut and sea lampreys. Otherwise these bile acids cannot be used as life-stage specific chemical cues. To the contrary, it seems very intriguing to find that some recently derived spe-cies release, even at their adult stage, the same bile acid as larval lamprey do considering they usually share the habitat with their larval stage conspecifics. There have been no stud-ies that identify PZS as a putative pheromone in the
In the Great Lakes, several strategies are used to control sea lamprey populations. The latest development is larval and sex pheromone research that uses pheromones as attractants to potentially enhance trapping efficiency (Li et al., 2003). Since some native species can produce both 3kPZS and PZS in their adult stage, application of bile acid pheromones to the natural environment poses possible ecological complications. There-fore, further systematic approaches are required to understand biological functions, geographical distributions, and phyloge-netic relationships of bile acids in the native lamprey species of the Great Lakes region.
To summarize, we have identified 3kPZS from mature male chestnut lamprey and PZS from mature male Pacific, river, and American brook lampreys. Different bile acid identities between the ancient and recently derived lampreys suggest that a modification of biosynthetic pathways for bile acid pro-duction has occurred during the course of evolution. Further systematic studies are required to better understand the evolu-tion of chemical communications among lamprey species.