Juvenile olive flounder
Biological indices of fish such as the hepatosomatic index (HSI) and condition factor (CF) haven been used to identify compensatory growth. HSI is a good compensatory growth index in rainbow trout
A relationship between hormonal changes in fish after fast-ing or refeeding has been reported (Eales et al., 1992; MacK-enzie et al., 1998; Gaylord and Gatlin, 2000). Additionally, MacKenzie et al. (1993) and Gaylord et al. (2001) reported that increase of plasma thyroid hormone is associated with an increase in growth rate of red drum
In the present we investigated the effects of dietary nutri-ents on the biological indices and serum chemistry of olive flounder achieving compensatory growth.
>
Fish and experimental conditions
Juvenile olive flounder were purchased from a private hatchery and transferred to the laboratory. Before initiation of the feeding trial, the fish were acclimated to the experimen-tal conditions for 2 weeks. In total, 450 fish averaging 16.0 g were randomly chosen and distributed into 18 180-L flow-through tanks (25 fish/tank). The flow rate in each tank was 6.5 L/min, and the water temperature was 16.0-25.5℃.
>
Preparation of the experimental diets
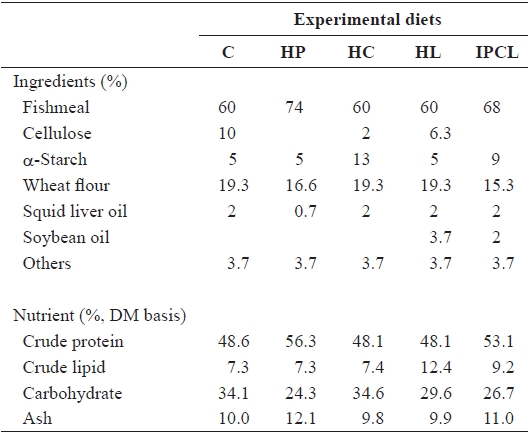
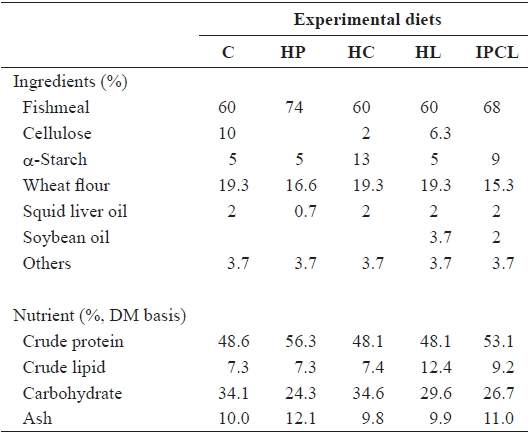
Five experimental diets were prepared: control (C), high protein (HP), high carbohydrate (HC), high lipid (HL), and intermediate protein, carbohydrate and lipid (IPCL) diets. The ingredient and nutrient composition of the diets are given in Table 1. Crude protein (48.6%) and lipid (7.3%) levels in the C diet satisfied the requirements for juvenile olive flounder (Lee et al., 2000, 2002). Protein, carbohydrate (nitrogen-free
[Table 1.] Ingredients and nutrient composition of the experimental diets
Ingredients and nutrient composition of the experimental diets
extract), and lipid levels in the HP, HC, and HL diets were increased to 56.3%, 31.6% and 12.4%, respectively, at the ex-pense of cellulose and/or wheat flour. Finally, protein and lipid levels of the IPCL diet were increased to 53.1% and 9.2%, respectively, at the expense of cellulose and wheat flour.
Six treatments were prepared in triplicate. Fish were hand-fed with the C diet to apparent satiation twice daily (09:30 and 17:00), 6 days per week, for 8 weeks (8W-C), or fish were starved for 2 weeks and then hand-fed with the C, HP, HC, HL, or IPCL diets to apparent satiation twice per day for 6 weeks, referred to as 6W-C, 6W-HP, 6W-HC, 6W-HL, and 6W-IPCL, respectively.
>
Analysis of biological indices and fish serum
Total length and body weight were measured in five ran-domly chosen fish from each tank at the end of the 8-week trial. Fish were dissected to measure liver weight to determine the biological indices: condition factor (CF) = fish weight (g)/total length (cm)3, and hepatosomatic index (HSI) = liver weight (g) × 100/fish weight (g).
Blood samples were obtained from the caudal vein of 5 ran-domly chosen 24-h starved fish from each tank by syringes. Serum was collected after centrifugation (3,000 rpm for 10 min) and stored at -70℃ in separate aliquots to analyze total protein, glucose, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), and triglyceride (TG) using automatic chemistry system (Vitros DT60 II, Vitros DTE II, DTSC II Chemistry System; Johnson and Johnson Clinical Diagnostics Inc., New York, NY, USA). Additionally, triiodothyronine (T3) and thyroxine (T4) hormones were also analyzed by radioimmunoassay and a gamma counter (Cobra II; Packard, Dallas, TX, USA).
A one-way analysis of variance and Duncan's multiple range test (Duncan, 1955) were performed to analyze differ-ences among the means of treatments using SAS program version 9.1 (SAS Institute, Cary, NC, USA). A
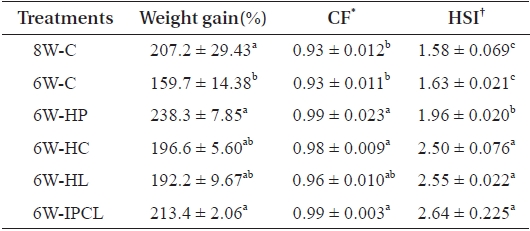
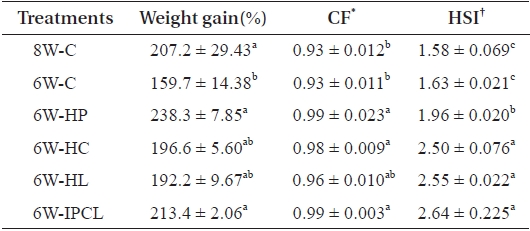
Weight gain (%) of olive flounder in the 8W-C, 6W-HP and 6W-IPCL treatments was significantly (
However, no significant difference in weight gain of all fish groups experiencing 2-week feed deprivation (6W-HP, 6W-HC, 6W-HL, and 6W-IPCL treatments), except for fish in the 6W-C treatment compared to that of fish in the 8W-C treatment indicated that supplementing diets with nutrient such as protein, carbohydrate, and lipids and their combi-nation resulted in compensatory growth. Moreover, a slight overcompensation of fish in the 6W-HP and 6W-IPCL treat-ments was observed suggesting that supplementing diets with protein and a combination of protein, carbohydrate, and lipids was effective to accelerate compensatory growth. Similarly, Cho and Heo (2011) showed that a combined HP (54.8%) and HL (14.0%) diet achieved overcompensation in juvenile olive flounder subjected to 1-week feed deprivation. Additionally, Gaylord and Gatlin (2001) showed that compensatory growth of channel catfish is primarily affected by dietary protein level rather than dietary energy level.
The CF of olive flounder in the 6W-HP, 6W-HC, and 6W-IPCL treatments was significantly (
Weight gain (%) and biological index of olive flounder Para-lichthys olivaceus fed the experimental diets containing various nutrient contents after 2-week feed deprivation
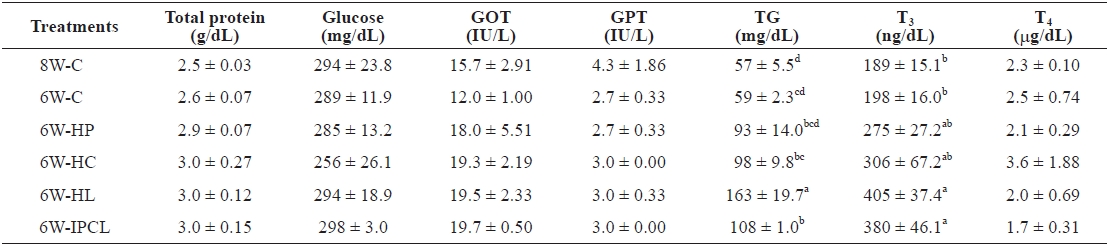
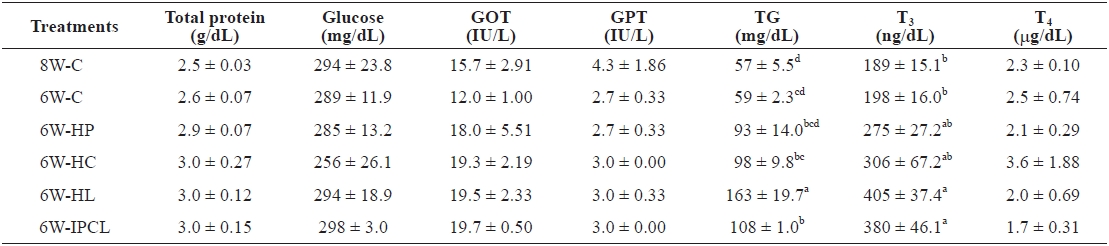
[Table 3.] Serum chemistry of olive flounder Paralichthys olivaceus at the end of the 8-week trial
Serum chemistry of olive flounder Paralichthys olivaceus at the end of the 8-week trial
of fish in the 8W-C and 6W-C treatments, but not significantly (
The HSI of olive flounder in the 6W-HC, 6W-HL and 6W-IPCL treatments was significantly (
Another reason for the high HSI in the 6W-HP, 6W-HC, 6W-HL, and 6W-IPCL treatments was probably due to the higher energy content of the diets (Table 1). This agreed with other studies showing that fish receiving higher energy diets have a higher HSI (Jobling, 1988; Nanton et al., 2003; Kjær et al., 2009). Kjær et al. (2009) demonstrated that a higher HSI in Atlantic cod (
Total protein, glucose, GOT, GPT, and T4 were not significantly different among the treatments (Table 3). However, TG of olive flounder in the 6W-HL treatment was significantly (
The T3 levels of olive flounder in the 6W-HL and 6W-IPCL treatments were significantly (
The results of this study demonstrated that CF and HSI of fish could be compensatory growth indices and that T3 seemed to play a partial role in achieving compensatory growth in ol-ive flounder.