Cryoprotectant agents (CPAs) are chosen because of their useful non-crystallization property of hydrate formation at low temperatures in aqueous solution (Wowk, 2010). How-ever, the toxicity of which is a fundamental limiting factor for the successful cryopreservation of living cells (Fahy, 2010). So far, most reports of CPA toxicities were focused on fin-fishes such as flounder embryos/sperm (Zhang et al., 2005), zebrafish (Zhang and Rawson, 1993; Liu et al., 2001), medaka (Yang et al., 2010), and so on. However, little research on the toxicity of CPAs to macroalgae has been conducted.
In order to determine the tolerance of gametophytic thalli of
>
Algal strains and culture conditions
Gametophytic thalli of
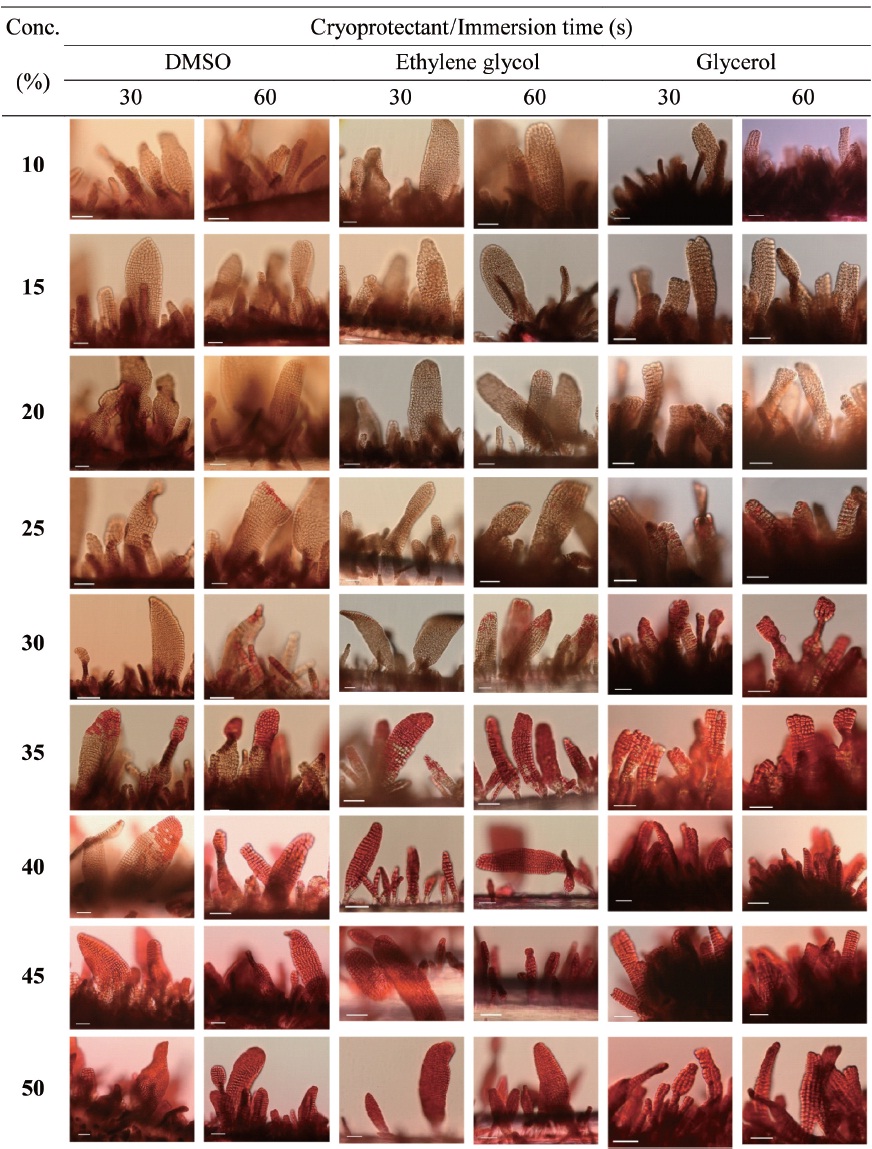
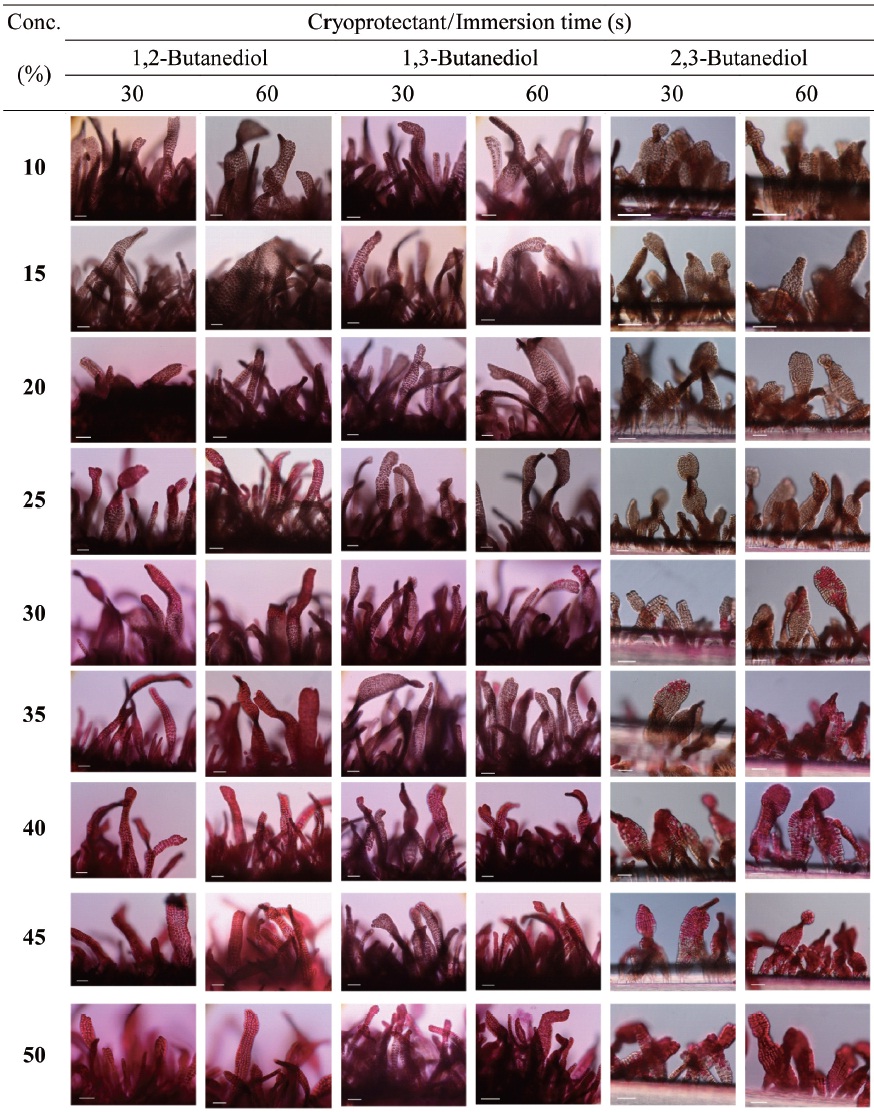
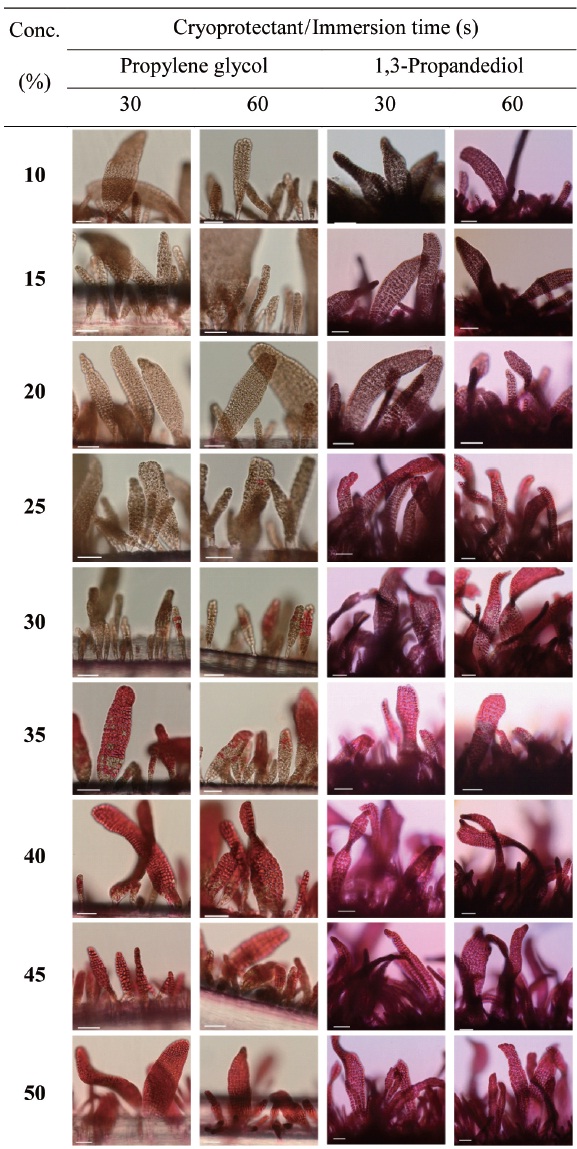
Nine concentrations (10, 15, 20, 25, 30, 35, 40, 45 and 50%) of each of the eight CPAs (DMSO, EG, GC, 1,2-BD, 1,3-BD, 2,3-BD, PG and 1,3-PD) were tested. All chemicals were re-agent-grade, and cryoprotectant solutions were prepared in 0.2 ㎛-filtered seawater. Synthetic fibers with attached thalli were cut to 0.5-1 cm lengths and transferred to CPA solutions for 30 or 60 s. Thalli were then stained with 0.1% erythrosine (in seawater) for 20 min and washed with fresh seawater.
Thalli viability was examined under a light microscope (BX-50; Olympus, Tokyo, Japan). The survival rates were ex-
pressed as the ratio of the number of living thalli to the total number observed.
Fig. 1 shows survival of the gametophytic thalli of
In aqueous solutions, 2,3-BD exhibits glass formation supe-rior to that of other CPA, including 1,2-PD and 1,3-PD (Bau-dot et al., 1996). Shaw et al. (1995) reported that propanediol forms a stable vitreous state on cooling to a greater degree than other CPA in human and mouse embryos. We found that gametophytic thalli of
Selection of a suitable CPA is important for successful cryopreservation. In general, DMSO, GC and methanol have been widely used in algae (Taylor and Fletcher, 1999). DMSO is the most-commonly used CPA for macroalgae,
et al., 2008). In this study, glycerol was less toxic than DMSO. Gwo et al. (2005) reported low glycerol toxicity in the micro-algae,
CPA toxicity depends on species, developmental stage and storage temperature. In particular, it is known that DMSO is less toxic at 0-5℃ than at higher temperatures (Hubalek, 2003). Therefore, the viability of thalli may be enhanced by low temperature preservation.
CPA toxicity occurs after permeating into the cell, so the more toxic CPAs are likely to be more membrane permeable, and