Myoglobin is a protein found in the muscle tissue of vertebrates in general and in almost all mammals. It tends to act as a sensitive marker for muscle injury, making it a potential marker for heart attack in patients with chest pain.1 In this study, myoglobin was investigated as a model protein.
For identification, proteins are often hydrolyzed into small peptide fragments using enzymatic digestion2,3 or chemical degradation,4 and microwave radiation has been applied as a means of accelerating both of these processes.5?11 A variety of other techniques have also been used to expedite protein digestion or to increase its efficiency, including pressure,12 vortex,13 and ultrasound.14 Diluted formic acid at high temperature reportedly cleaves proteins predominantly at the C-terminus and occasionally at the N-terminus of aspartic acid residues.15, 16 Recently, microwave irradiation was used to expedite formic acid cleavage at Asp residues for high-throughput proteome analysis.17
In this study, myoglobin from horse heart was hydrolyzed by microwave-assisted weak acid hydrolysis using 2% formic acid at various temperatures (37 ℃, 50 ℃, and 100 ℃) for 1 h. For comparison, the same hydrolysis procedure was performed in boiling water at various incubation times (20 min, 40 min, 60 min, and 120 min).
All chemicals were purchased from Sigma (St. Louis, MO, USA) unless otherwise noted. A stock solution of 10 mg/mL (5.9 × 10-4 M) horse heart myoglobin was prepared in deionized water. For weak acid hydrolysis, 10 mL of the stock solution was mixed with a solution of 2% formic acid (2 mL formic acid and 88 mL deionized water) and placed in a microwave oven (HST Rapid Enzyme Digestion System with 800W output at 60 Hz, AC 220?240 V) from Hudson Surface Technology (Fort Lee, NJ, USA) at various temperatures (30 ℃, 50 ℃, and 100 ℃) for 1 h. As a control, the same protein solution was heated in a boiling water bath for various times (20, 40, 60, or 120 min).
To prepare the matrix solution for matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analyses, 10 mg of 2,5-DHB was dissolved in 1.0 mL of 50% ACN/0.1% TFA in water. A two-layer sample preparation method was adopted for analysis of the hydrolyzed myoglobin peptides in which 1 μL of matrix solution was placed on a MALDI plate and allowed to dry, forming a microcrystal layer. Then, 1 μL of the protein solution mixed with the matrix solution (1:1 by volume) was spotted on top of the microcrystal layer. Each MALDI spot contained 29 pmol of hydrolyzed myoglobin peptides.
All mass spectra were obtained on a KratosAxima CFRTM (Shimadzu Biotech, Kyoto, Japan) time-of-flight mass spectrometer equipped with a 337-nm nitrogen laser operating in positive reflectron mode. Each mass spectrum was a result of summing 100 laser shots with laser intensity of 120 (arbitrary unit).
Peptide identification was performed using the Swiss-Prot database, FindPept program (http://web.expasy.org/findpept/), and PeptideMass program (http://web.expasy.org/peptide_mass/) with an enzyme option for microwaveassisted formic acid hydrolysis (C-term to D). The information of horse heart myoglobin can be found using at UniProt (http://www.uniprot.org) with the Swiss-Prot accession number/entry name of P68082/MYG_HORSE.
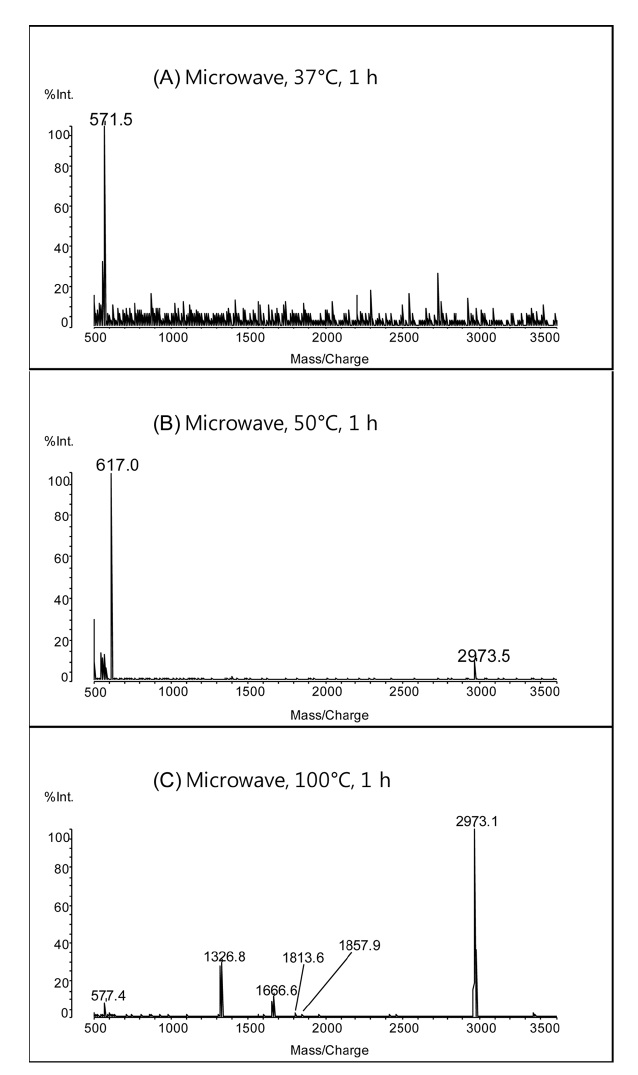
In horse heart myoglobin, there are 8 aspartic acid residues. Complete C-terminal cleavage of aspartic acid residues using weak acid-hydrolysis provides 9 peptide fragments. Figure 1 shows MALDI mass spectra of peptides obtained by the microwave-assisted hydrolysis of myoglobin in 2% formic acid at 37 ℃, 50 ℃, and 100 ℃ for 1 h. The peak at
increased from 37 ℃ to 100 ℃. The presence of more cleavage products indicates that higher temperatures enhance the efficiency of microwave-assisted acid hydrolysis of proteins.
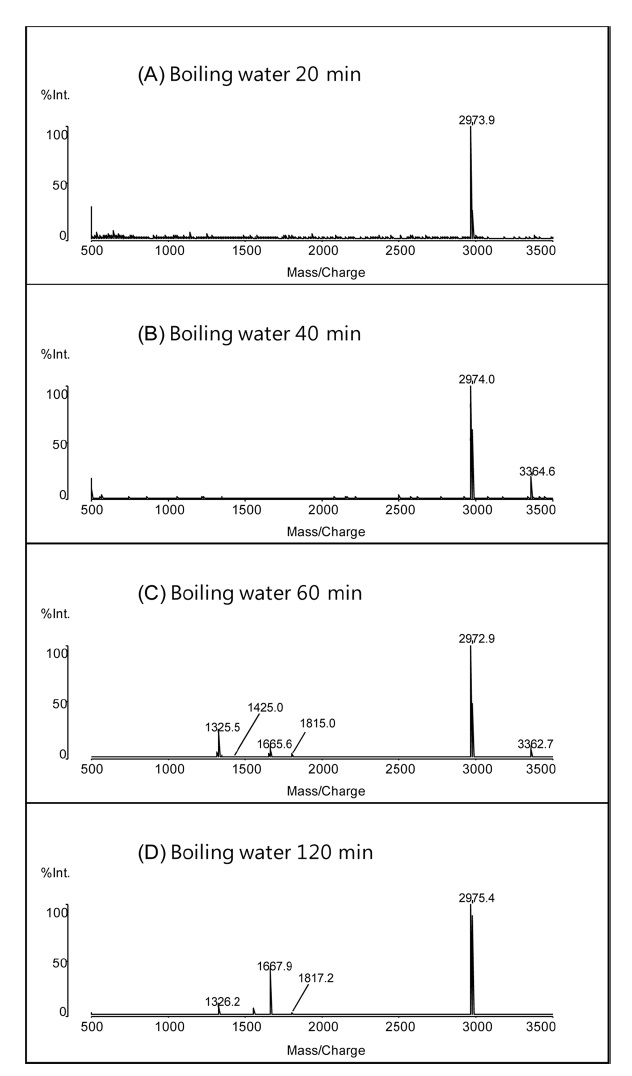
The same hydrolysis procedure was also performed in boiling water without the application of microwave radiation. Figure 2 shows the MALDI mass spectra peptides obtained by weak acid hydrolysis of myoglobin in 2% formic acid in boiling water for 20, 40, 60, and 120 min. The number of the peptide peaks increased with hydrolysis time up to 60 min. After 120 min, two additional differences were observed in the mass spectra: the disappearance of a peptide with two missed cleavages at
Summary of the identified weak acid-cleaved peptides from myoglobin using microwave and boiling water under different conditions.
observed at the MALDI mass spectrum obtained by the weak acid hydrolysis for 120 min.
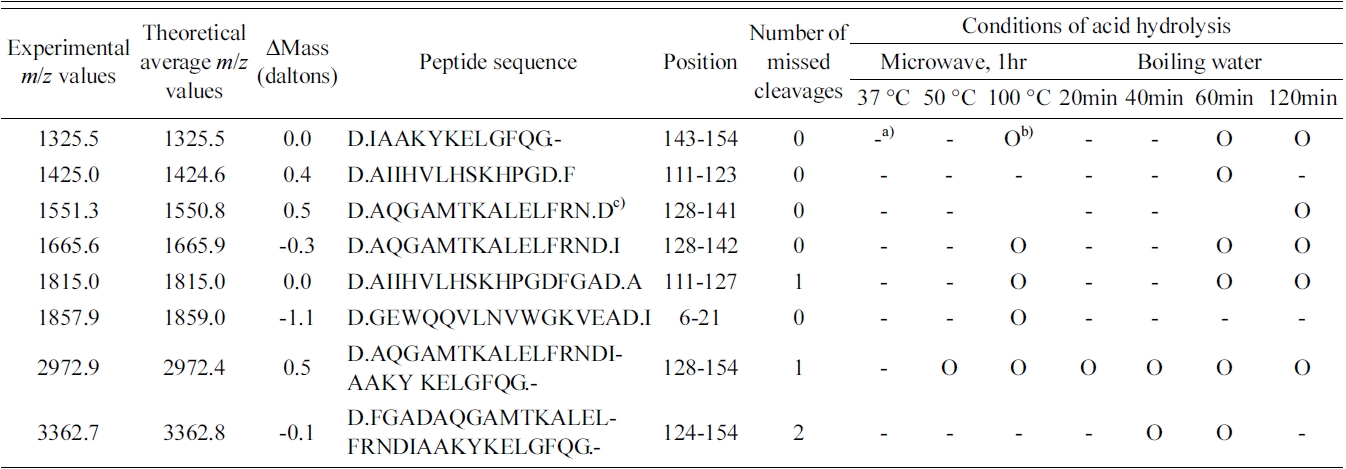
Table 1 shows the summary of the identified peptides from myoglobin using microwave and boiling water at various conditions. A peptide with amino acids 111?123 was only identified at the MALDI mass spectrum obtained by the weak acid hydrolysis for 60 min, while a peptide with amino acids 6?21 was only observed at the MALDI mass spectra of peptides obtained by the microwave-assisted hydrolysis at 100 ℃ for 1 h. The intensities of both of the two peaks were found to be very small. In general, the MALDI spectra obtained by microwave-assisted weak acid hydrolysis at 100 ℃ for 1 h were very similar to those acquired following the same hydrolysis for 1 h in boiling water. This suggests that microwave irradiation has no notable enhancement effect on the weak acid hydrolysis of proteins.
Similar results were obtained for the weak acid hydrolysis of myoglobin at 100 ℃, regardless of microwave assistance. This suggests that heat was the primary catalyst for the enhancement of weak acid hydrolysis efficiency and that microwave irradiation provides no additional enhancement.