High birefringence nematic liquid crystals (NLC) have been researched broadly to satisfy need of an extensive optical phase shift upon driving voltage in infrared range (IR) photonics devices.Examples of such are diphenyl-diacetylene LCs [1,2], bistolane [3,4], naphthalene tolanes [5], and thiophenyl-diacetylene [6-8]. Although proven possible to reach values of the optical anisotropy (Δn) of 0.5-0.7 within visible range of spectrum, typically these materials carry on intrinsic limitations for their application potential. More recently, ultra high birefringence structures combining naphthalene, thiophenyl-diacetylene, and bistolane building blocks were synthesized, evaluated and discussed from the mixture formulation stand point [9,10]. Although NLC mixture with Δn of 0.6-0.7 was successfully achieved, intrinsic high rotational viscosity overshadowed the merit of birefringence. Therefore, the above presented approaches resulted in high birefringence material proven not applicable due to either excessive viscosity or thermal and photostability issues. In this paper we introduce the high birefringence nematic LC single compounds and mixtures with birefringence of 0.50 at visible range and the rotational viscosity similar to popular E44 and BL038 materials (Merck Inc.)
Several measurement techniques were used to assess the physical properties of the single compounds and mixtures. A Differential Scanning Calorimetry (DSC, SETARAM) was used to determine the phase transition temperatures. Results were obtained from 3-6 mg samples in the heating and cooling cycles with rate 2℃/min. The electro-optic properties of the LC com-
[Table 1.] Terphenyl compounds with Δn within the range 0.35-0.37 [1213].
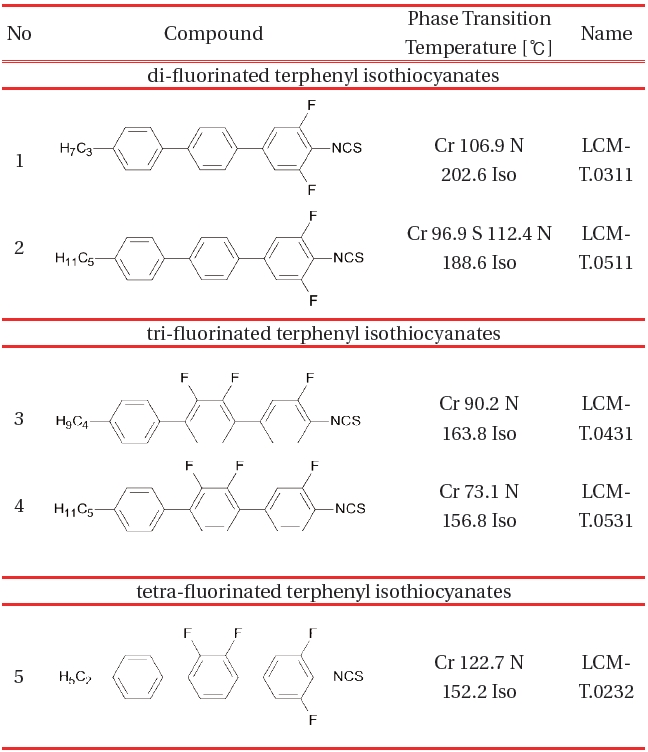
Terphenyl compounds with Δn within the range 0.35-0.37 [1213].
pounds and mixtures were measured using an 8 μm homogenous cell with ITO (indium-tin-oxide) electrodes coated in the inner sides of the glass substrates. A thin polyimide layer was overcoated on ITO and buffed in anti-parallel directions to produce a small pretilt angle (~2°). A linearly polarized He-Ne laser with λ=633 nm was used as the light sources for the electro-optic measurements.
The phase retardation (δ) of the homogeneous cells was measured by the LabVIEW system. The LC birefringence (Δn) at wavelength λ and temperature T is obtained by measuring the phase retardation (δ) from the following equation [11]:
[Table 2.] Phenyl Tolane compounds with Δn within the range 0.45-0.51 [14-16].
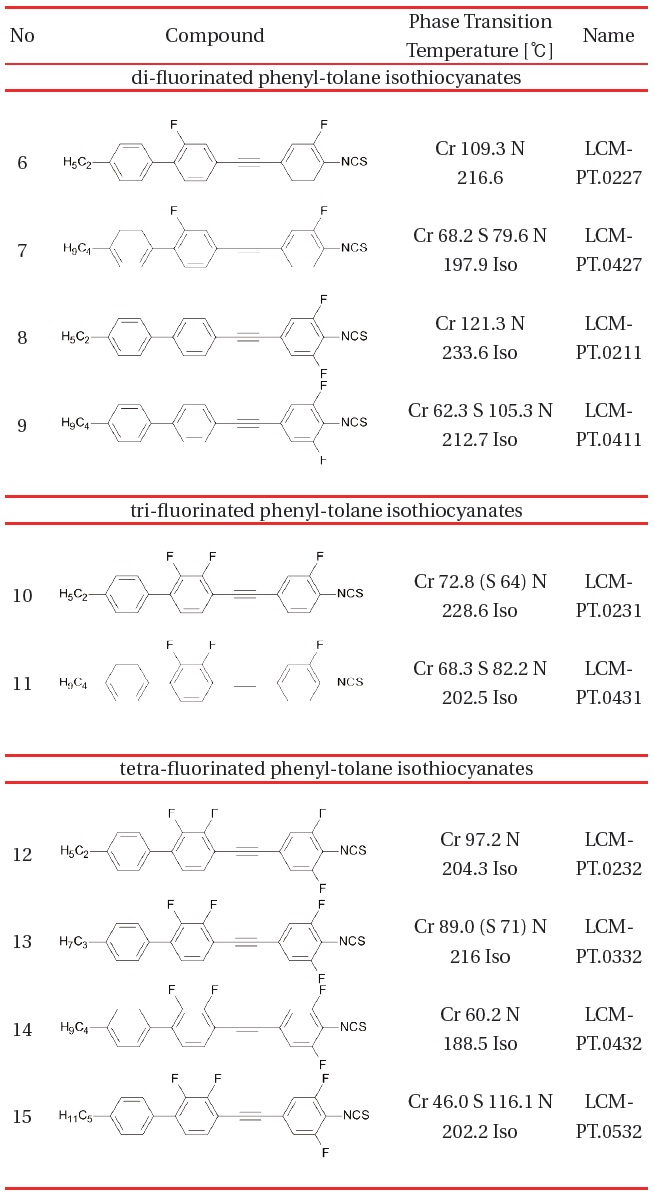
Phenyl Tolane compounds with Δn within the range 0.45-0.51 [14-16].
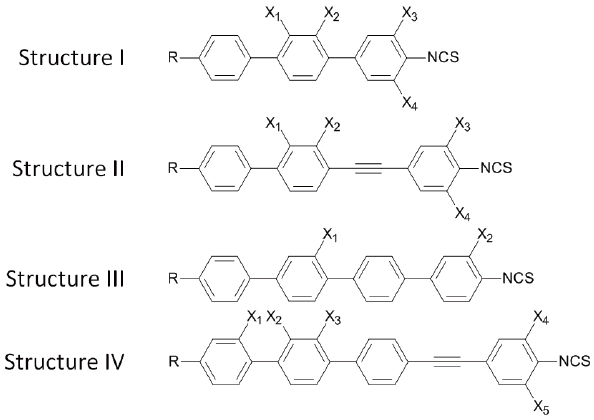
The structures and phase transition temperatures of the single compounds utilized in this work are listed in consecutive tables of this paragraph. The condition is the length of the molecular rigid core. Given that it is designed to maintain π-electron conjugation along long molecular axis, there are terphenyl, phenyl tolane, quarterphenyl, and biphenyl tolane type of molecules used in our work, general structure I-IV respectively.
Typically, increasing the length of the rigid molecular core, while maintaining the conjugation and without excess lateral substituents increases the density of the molecular packaging. Therefore, the melting point temperature and the tendency to build smectic phase instead of nematic severely increases. Both are unfavorable for mixture formulation. Multiple fluorination and/or alkylation in lateral positions of the rigid core can effectively reduce the unwanted consequences of elongated molecular rigid core. Although the melting point temperature can be re-
[Table 3.] Four rings compounds with Δn exceeding 0.60 [1718].
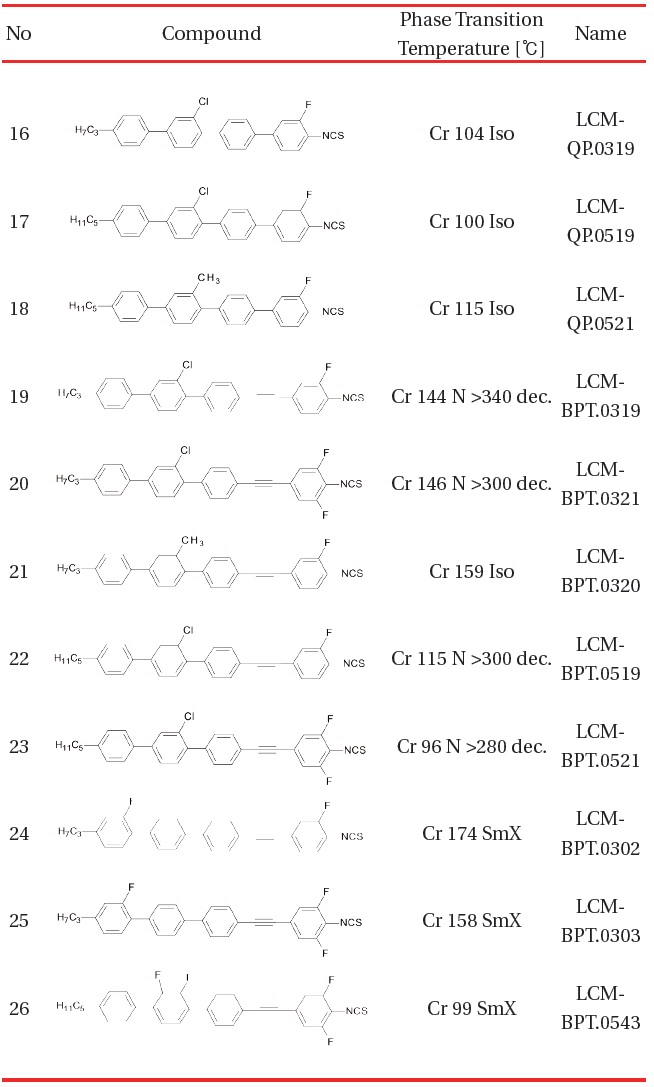
Four rings compounds with Δn exceeding 0.60 [1718].
duced, there is also a tradeoff by slight decrease in birefringence of the multifluorinated compounds as compared to their nonfluorinated analogues.
The Table 1 shows the first group of the compounds, the homologues of a terphenyl general structure I. Multiple lateral fluorination effectively suppresses the melting point temperature.
The density of the molecular packaging decreases as the number of lateral fluorine increases. Macroscopically this is manifested by lower transition temperatures, especially clearing point temperature. The melting point temperature depends also on the length of the alkyl terminal therefore the direct comparison is not adequate.
The second group of high birefringence compounds is shown in Table 2. These are homologues of general structure II. Although the length of the rigid molecular core increases by ethynyl bridge to form phenyl tolane structure, the lateral fluorination suppress the melting point temperature to the level of similar to terphenyl compounds Table 1.
Multiple fluorination leads to decreased melting point temperature and suppressed smectic phases which are often present for phenyl tolane isothiocyanates. The maximum birefringence we have been able to obtain for the formulations based on the terphenyl and phenyl tolane isothiocyanates does not exceed 0.45 when measured at 23° and wavelength of 633 nm. The example of such material is shown in Table 4. Therefore, compounds with
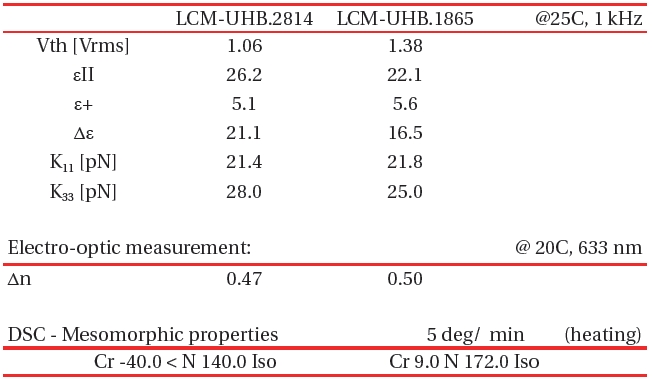
[Table 4.] Basic Properties of LCM-UHB LC mixtures.
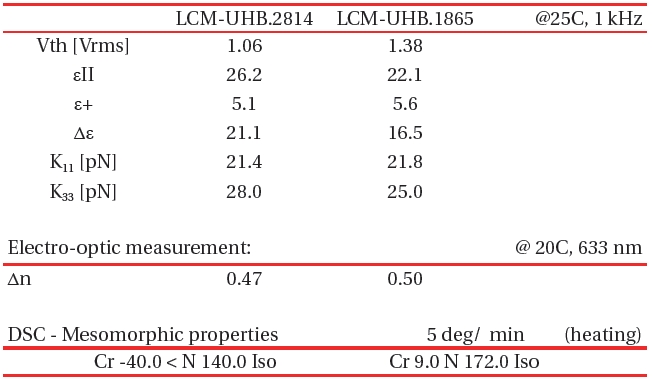
Basic Properties of LCM-UHB LC mixtures.
longer conjugation need to be implemented. Table 3 shows four rings compounds, the general structure III and IV.
The phase transition temperatures of four rings compounds clearly demonstrate the challenge of expanding the π-electron conjugation along the molecular long axis. The quaterphenyl based compounds do not show any mesophase despite neither the length of the alkyl terminal chain or lateral substitution. Introduction of the triple bonds in the way which makes molecular core asymmetric (biphenyl tolane structure) easy the packaging density and allow the nematic phase in exceptionally wide temperature range. In fact, we have not observed the transition from the nematic to isotropic state but rather a material decomposition as temperature exceeds 300℃.
3.2 Ultra high birefringence nematic LC mixture
Utilizing high and ultra-high birefringence compounds described in Table. 1-3 we have designed and tested number of different formulations with the aim at highest possible birefringence but also nematic temperature starting at 0℃ or below. Table 4 shows the examples of two high birefringence LC mixtures optimized for low melting point temperature. The melting point of LCM-UHB.2814 mixture is below -40℃. A drawback is that the maximum birefringence for this material was compromised by excess amount of low molecular weight component in order to suppress the smectic to nematic phase transition temperature. The second mixture in Table 4, (LCM-UHB.6814) is a formulation optimized for high birefringence with nematic range narrowed by the transition from nematic to a smectic phase at 9℃. Table IV lists physical properties of both mixtures.
Mixture LCM-UHB.1865 besides having a drawback of smectic to nematic transition at 9.0℃ shows clearing point at a 172℃which extends the nematic range for over 160 degree. The rotational viscosity of this mixture remains relatively low at ~530 mPa*s which is typical for materials like E48 or BL038 (Merck Inc.) having only half of the birefringence of LCM-UHB.1865 material.
We have successfully developed high and ultra-high birefringence molecular systems based on multi-ring rigid cores. Extended π-electron conjugation allows the birefringence ranging from 0.37 to 0.70 respectively for three rings terphenyl and four rings biphenyl tolane isothiocyanates. Relatively high melting point temperature of highly conjugated compounds prevents us from using four rings quarterphenyl and biphenyl tolanes as the major part of the mixtures. A careful balance between three rings terphenyl, phenyl tolanes and four rings compounds has to be maintained in order to achieve the nematic phase at the temperatures below room temperature while maximizing the birefringence.
The two examples of the mixtures presented in this paper illustrate the feasibility of the nematic liquid crystal having birefringence of 0.50-0.51 (at 23℃, 633/589 nm) which are thermodynamically stable. Unlike our previous approach documented in Ref. 9, the current material does not crystalize and maintain the original physical properties when stored for extended time.
We understand that this type of nematic LC with high birefringence is beneficial for photonics applications in infrared region of the spectrum. Ones like laser beam steering, light shutters or scene seekers systems are especially sensitive for the response time which can be significantly shorter when ultra-high birefringence and medium viscosity nematic is applied to minimize the required cell thickness.
![Terphenyl compounds with Δn within the range 0.35-0.37 [1213].](http://oak.go.kr/repository/journal/11428/E1TEAO_2012_v13n1_2_t001.jpg)
![Phenyl Tolane compounds with Δn within the range 0.45-0.51 [14-16].](http://oak.go.kr/repository/journal/11428/E1TEAO_2012_v13n1_2_t002.jpg)
![Four rings compounds with Δn exceeding 0.60 [1718].](http://oak.go.kr/repository/journal/11428/E1TEAO_2012_v13n1_2_t003.jpg)