Process control in semiconductor manufacturing is crucial for achieving the ultimate goals of higher product quality and reliability with lower manufacturing cost. As circuits and devices are highly integrated with the advent of 45 nm or below, tighter process control plays a very vital role to maximize manufacturing efficiency by minimizing misprocessing. Mostly plasma process control was based on the statistical methods with tool and metrology data, but advanced process control (APC) has recently emerged as a promising technique to improve the capability of process control for cost-effectiveness and higher yield [1]. By utilizing in-situ process monitoring technique, overcoming challenges in APC has become feasible and this includes real-time process fault detection.
Semiconductor manufacturing requires numerous process steps for the successful fabrication of integrated circuits (ICs), and more than 40% of the unit processes employ plasma processing in both the front-end-of-line (FEOL) and as well as in the back-end-of-line (BEOL). In the current semiconductor manufacturing, the importance of plasma processes cannot be over-emphasized while APC in the plasma process with realtime monitoring and analysis becomes increasingly important for both the current and future semiconductor manufacturing. Various in-situ sensors are used for the monitoring of plasma processes. In order to detect the electrical character of plasma, electrical devices, such as self-excited electron resonance spectroscopy (SEERS) and IV-probes have been used [2,3]. Plasma process monitoring which uses optical emission spectroscopy (OES) has also been suggested as non-invasive monitoring [4].
OES has been widely used for the monitoring of plasma process. OES measures the optical emission intensity from the glow discharge of reactive species of plasma and it provides useful information about the plasma physics and chemistry. The most well-known application of OES is end-point-detection (EPD) in etching by tracing one or more specific wavelengths and these wavelengths correspond to the etch by-products along time [5]. Moreover, it is also reported that OES can be utilized as a useful tool for in-situ real-time process fault detection and classification [6]. Despite the usefulness of OES, the selection of the corresponding wavelength peaks is arduous and this is due to representation of complicated plasma information which includes chemical species densities, electron density, and electron temperature that is in a wide range of optical spectrum. Moreover during plasma etching, it is also difficult to identify a single wavelength peak that corresponds to a specific gas due to the multiple presences of related peaks with same type of a gas molecule. For instance, we may observe more than 200 optical emission peaks that are carbon and fluorine related and they contain species from tetrafluoromethane (CF4). In general, the selection of the appropriate wavelengths for process monitoring is done by experience in engineering. Recently, investigations have been carried out on the principal component analysis (PCA) of OES data and this supports the engineering insights in the selection of the significant peak lines of OES data set by reducing the dimensionality [7]. However, quantitative analysis of OES data still remains a known concern. The detected emission intensity is the photon-count in the charge coupled device (CCD) type detector. In fact, the magnitude of optical emission intensity is a function of the distance between the end of an optical fiber and the view port on the chamber.
In this paper, for the analysis of OES data we present a wavelength selection method. This method uses standard deviation and correlation coefficient, and moreover, the intensities of the selected peaks are normalized by that of mostly suggested Ar peak, for actinometry [8]. The suggested method is applied for the analysis of abnormal etching of silicon oxide which we had previously experienced. During a series of etch experiments in this endpoint detection research, we had an abnormally etched result while a few tens of identical timed-etch runs were conducted in a short period. In the whole experimental period, the fault appeared in the first quarter and no noticeable perturbation was observed..
By investigating on the data set by reactant and by-product species, separately, from the difference of abnormal etch from the baseline. Then, we found out the evidence of erosion of photoresist etch mask and this was the primary cause of the abnormal process. However, the proposed analysis was conducted offline and the suggested method can be applied for in-situ realtime monitoring of SiO2 etch process. Moreover, it is also ready for practical utilization in other engineering facilities. Section 2 describes the suggested wavelength selection method and the chemical actinometry that was performed. Section 3 presents the analytical results of OES data, OES data analysis was used as a proof for the suggested wavelength selection method and this is followed by the conclusion in Section 4.
Utilization of OES for plasma process monitoring requires corresponding wavelength selection. This selection is done for the analysis of chemical species and physical/chemical behaviors of gas species that are found inside the process chamber. Empirical selection of the emission spectral peaks in by-product of etch process is the most commonly used, and timely tracing of emission intensity of SiF peak line in silicon oxide etching is an example of the end point detection (EPD). However, one chemical species may present more than a few well-known emission lines, and this can add to the complexity in the selection of the corresponding wavelength in the mixtures of reactant and by-product gas species. In order to alleviate the concern, statistical methods, such as principal component analysis (PCA) and partial least squares (PLS), have also been suggested to reduce the data dimensionality of the acquired OES data set [9]. Both PCA and PLS are based on the feature extraction in a multi-dimensional data set and this is done by the elimination of the insignificant variations that are innate in a given data set. Then, the extracted features are regarded as the dimensionality-reduced data set which maintains most of the variations of the original data set and they are within the selected few variables. PCA and PLS are proven to be useful for the reduction of multi-dimensionality problems, and moreover, they have been employed in the selection of statistically significant variables in the OES data set. Selection of statistically significant variables is mostly correct to reflect the variations of OES data during the process. Furthermore, how the statistical significance is related to the physical phenomenon of plasma emission intensity remains as a question. In other words, including peaks that are physically less meaningful but have larger variations may result in a dominant peak in the extracted features without reflecting physical phenomenon in plasma process. In order to avoid such a misleading, engineering principles are to be employed before any statistical treatment of the given data set.
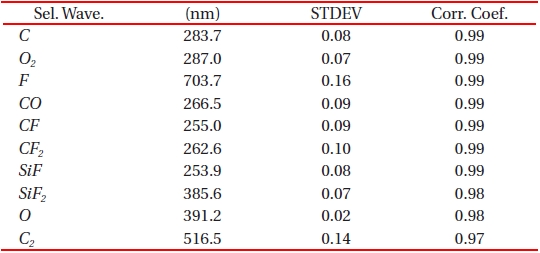
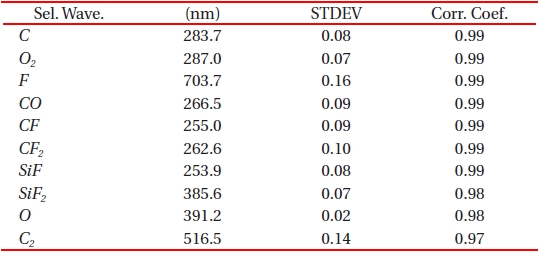
In silicon oxide etching, wavelengths may be grouped into reactant (or feeding) gases and by-products and this is done according to the mechanism of reactive ion etch. Moreover, as etching progresses the amount of ionized fluorine atoms in the chamber gradually decreases and they become saturated at some point while silicon oxide film is consistently removed. Meanwhile, the behavior of the by-product, such as SiF, shows opposite to the reactant gas species. When no more material to be etched remained, compared to the by-product, the reactant gas becomes dominant gas species. The purpose of separating the by-product from the reactant gas is to explain most of the variance in the original data set but at the same time without resulting in too much loss of chemical information. We compared both the standard deviation and correlation coefficient of the selected relevant wavelengths in both reactant and by-product. By using standard deviation in each group, we eliminated the wavelengths whose variations of emission intensity were small enough. This enables to include information from both reactant gas and by-product. A standard deviation provides a selection of the largest variation for the stepwise-increased reactant gas and also the determination of wavelengths that contain less distortion from noise. Correlation coefficient was also implied to find out a relationship between the increase in the source gas and the increase in the emission intensity. A correlation coefficient which is close to 1 or -1 suggests a strong relationship between the two groups. It proves the appropriateness of the selected wavelengths. Correlation coefficient was also calculated by using the following equation,
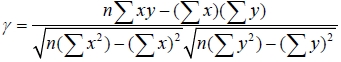
Where, n is the number of acquired data points of the OES spectrum. Among more than 200 relevant spectra lines that were taken in to consideration, we were able to select ten most relevant wavelengths and they are as shown in Table 1.
In a plasma process, Ar actinometry is a method that uses a uniform flow of Ar. Atomic Ar exists independently in the plasma state, and moreover, it does not affect the plasma chemistry due to inertness. Using actinometry herein with Argon, it also provides normalization of radical emission intensity to eliminate the effect for the change in the electron temperate and electron density from various gas species in the chamber. Optical emission intensity that is acquired from OES is a function of electron temperature Te, number of electrons ne, and molar fraction of input gas N. It can be represented as IN= k(Te)neN. Any fraction of the simultaneously acquired optical intensity can eliminate the effect of electron temperature and electron density, and it only remains as the molar fractions of gas species which are observed as follows,
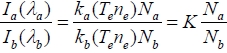
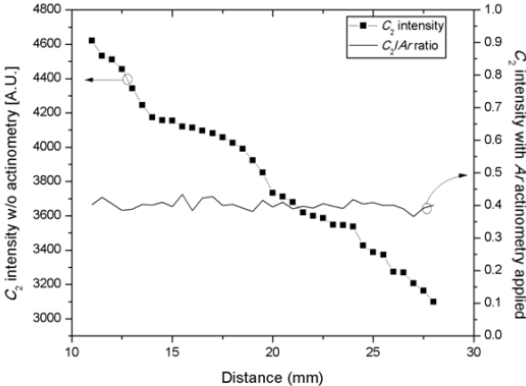
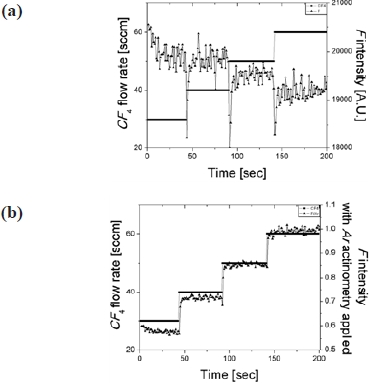
The optical emission intensity from plasma emission is the representation of photon counts at the detector, and it is true that the magnitude of intensity is a function of the distance that is found between the mounted optical fiber end and the sidewall viewport. In this research, in order to eliminate this dependency and to support engineering principle for the analysis of OES data, we employed Ar actinometry. The necessity of Ar actinometry for the quantitative analysis of OES data in this research is shown in Fig. 1. When a test etch run was running, the location of the end of the optical fiber was continuously dislocated from the
sidewall viewport. It is straight forward that the intensity of C2 which is one of the gas species that is monitored during the process continuously decreased along the increased distance from the viewport. However, the intensity that applied Ar actinometry, presents reasonable stability in its intensity ratio, and this shows the usefulness of chemical actinometry for the selection of OES peaks that corresponding to the process variations.
Before the adoption of the chemical actinometry for the photochemical conversion of OES data set, we verified the usefulness of Ar actinometry for plasma etching monitoring. A test etch run was conducted using an inductively coupled plasma (ICP) type reactive ion etch (RIE) in the MINIPLASMA-Station, manufactured by Plasmart Co. Ltd. Korea, while in-situ OES was collected in real-time. Engineering principle that was employed in this experiment is Ar actinometry and this was used for the analysis of OES data which was acquired from silicon oxide etching process. The split experiment consists of four levels of CF4 flow from 30 to 60 sccm with 5 sccm of Ar and throughout the experimental run 500 watts of 13.56 MHz RF power was applied while the base pressure was maintained at 10 mTorr. In order to monitor the plasma optical emission, the optical fiber was mounted on a sidewall viewport and the detection range was about 180 nm to 990 nm. During a test experiment, Fluorine peak intensity was monitored with the stepwise increment of CF4 gas flow. Optical emission intensity of F species exhibited no significant correlation with the increment of the reactant gas flow and this is shown in Fig. 2(a). By applying Ar actinometry, the properly scaled intensity showed dramatic accordance with the reactant gas flow modification. This is shown in Fig. 2(b). From the Figs. 2(a) and 2(b), we are convinced that the actinometric study is beneficial for the OES data analysis. Moreover, the practical utilization of the suggested method is presented in the next section.
In order to probe the usefulness of the emission actinometric wavelengths selection method, we have re-visited in-situ OES data which was acquired from series of experiments that were previously conducted of silicon oxide etching. ICP-RIE with 13.56 MHz RF power system was used for the etching of thermally grown silicon oxide with 50 sccm of CF4 and 3 sccm of Ar. While performing the experiments for etch end point detection, we collected in-situ optical emission spectrum via sidewall view-
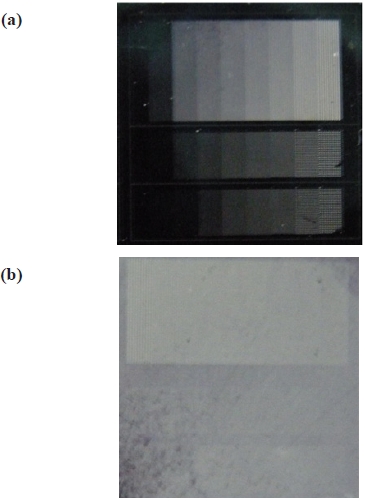
port with 10 times of integration for every 200 micro seconds. A series of etch experiments were conducted under the same etch condition and even though none of the process conditions were changed, we observed that a couple of samples were abnormally etched. Figure 3 shows two etched samples where, the one in the left is a normally etched sample and the other on the right is abnormally etched. They have line and via pattern etch in their size of 5-50 μm with AZ1512 positive photoresist mask. During the experiment no noticeable anomaly was observed and recorded. So, we applied the suggested actinometric analysis for the preselected in-situ acquired OES data. As described in the previous section, we included both the reactant and the by-product gas species along with engineering principles in plasma etch chemistry. As soon as the OES peaks for the analysis were determined, Ar actinometer was applied to the selected OES peaks for the normalization of radical emission intensity of the plasma gas species.
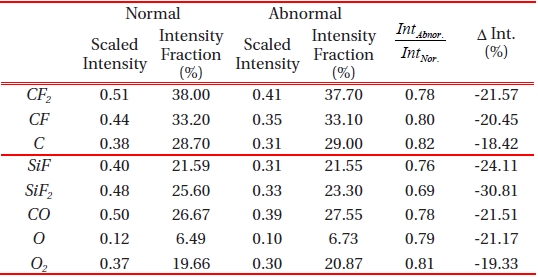
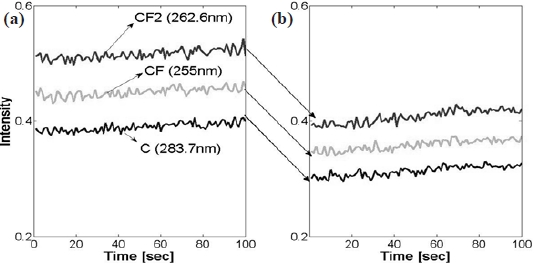
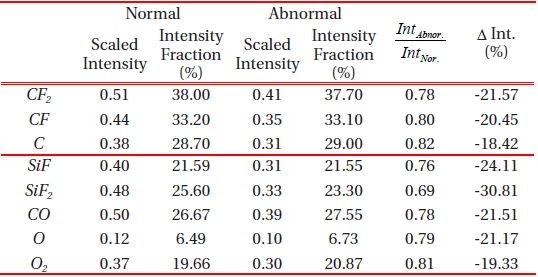
Emission actinometric analysis was performed by comparing the observed intensity of C, CF, and CF2 in their magnitudes from two data sets; normal and abnormal process. Plasma processing uses CF4, as CF3, CF2, and CF radicals. Moreover, the ions that result from the electron impact ionization of CF4, are one of the most abundant and reactive species. As the cross section for the molecular fragments (CF2+, CF+ from CF3) exceeds the parent ionization cross section of CF3+ from CF3 [10], the molecular frag-
ments of C, CF and CF2 were taken into accountfor the actinometric intensity comparison of the two type of process as provided in Table 2. The Ar actinometry intensity fraction of the acquired optical emission intensity shows the percentage of molar fraction of the corresponding chemical components in the chamber, and we consider it as a portion of the dissociated reactive ions in the plasma. In both cases of normal and abnormal etching, no significant difference in the chemical dissociation ratio of the fraction of CF4 was found from the two cases. However, the actinometry intensities of the fragments in abnormally etched process were decreased by approximately 20% in their magnitude. It seems that the intensity fraction in terms of plasma chemistry was not the first issue that was taken in to consideration. Moreover, other physical phenomenon that is related to electron impact ionization is also to be considered for further analysis. This postulation is based on an overall decrease in the electron cross section during the abnormal etch run, and this is also illustrated in Fig. 4.
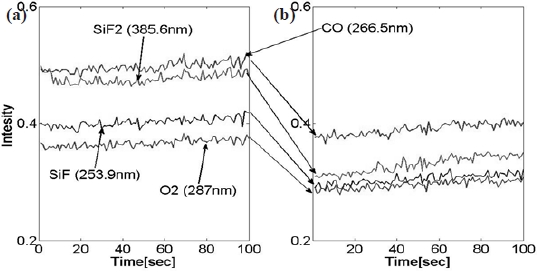
One may simply assume that the decreased optical emission intensity is associated with the reactant gas species and can be explained with the increased emission intensity of the byproduct. On the contrary, we observed was that the emission intensity of the by-product also decreased. SiF, SiF2, CO and CO2 are known as the most significant volatile etch by-products in silicon oxide, and their lower level of emission intensity in abnormally etched process, by approximately 23% in its magnitude, is presented in Fig. 5. Analysis on the by-product provided plausible evidence which showed that abnormally etched process is different from the normal baseline process. Monitoring the actinometry intensity of the by-product is useful for real-time sensor based-advanced process fault detection. However, it is still undercover that what caused the electron interactions with CF4 neutral fragment. Furthermore, it is required to investigate on the electron-impact dissociative ionization cross section of
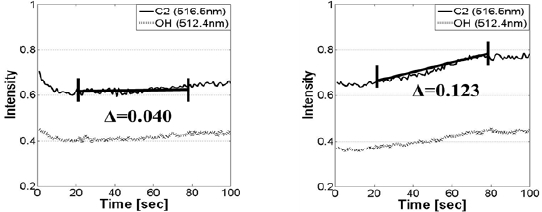
the plasma to form C+ and F+ fragment ions, but instead we, have investigated on the emission intensity of C2 and OH of the abnormal sample because the microscopic image showed blurred image and this is correlated with photoresist etch mask erosion. In silicon dioxide etching with photoresist mask, fluorine containing gas species without or very small amount of oxygen is generally used feeding gas chemistry to increase SiO2 etch rate. Fluorine radical forms volatile SiF, and carbon helps to form CO. Oxygen decomposed from SiO2 can also be ionized during the process, and it may react with polymer photoresist to cause the etch mask to be etched. Generally, as the total amount of oxygen supplied in the chamber is limited, the increment of emission intensities of C2 and OH from photoresist is unexpected. In our anomaly we have observed gradual increment of the intensities that are associated with photoresist, by three times larger in their slope than in a normal process. It is obvious that this observation indicates photoresist erosion during the etching and this is shown in Fig. 6.
Optical emission spectroscopy (OES) is utilized for the analysis of abnormal etching of silicon dioxide by direct comparison with the normal process in several ways. We suggested practically simple and useful method for the selection of high dimensional OES data with significant wavelengths. By employing engineering principle before the application of previously established statistical techniques, emission peaks of reactant and the byproduct gas were separated from each other. Moreover, chemical actinometry was applied for the normalization of the radical emission intensity to and also for the elimination of the effect for the change in the electron temperate and electron density from various process gas species in the chamber. Our postulation for the lowered electron-impact dissociative ionization from this analysis as the root cause of the abnormal etch process, still remains as some extent of investigation in plasma physics. However, we report that the acquired optical emission intensities of gas species inside the etch chamber during real plasma etching are not from a confined physical experiment, is related with that of additionally created etch by-product, and they are inter-related. Utilizing optical emission spectroscopy for plasma monitoring has been useful for numerous applications, but chemical actinometry should be engaged to make emission intensity information in practical use for quantitative plasma process analysis. We remark that the chamber condition as well as RF power energy is critical in silicon oxide etching and a fragment of it which is delivered, is extremely essential in the dissociation of tetrafluoromethane (CF4)]. The choices of gas species are many, but CF4 is also useful for plasma etching of other materials, such as silicon nitride and low-k dielectrics, such as SiLKTM in semiconductor fabrication. Therefore, the contributions of this research can be expanded to real-time in-situ monitoring of dielectric material etching in semiconductor manufacturing.