In order to provide renewable green energy sources, many methods have been tried. Among these, the rechargeable Li ion battery has attracted a great amount of interest. A Li ion battery has the ability to store and reversibility release electric energy with high density. It is used in many fields, such as powered portable devices like mobile-phones or laptops, and power sources for electrical vehicles and hybrid electrical vehicles. The Lithium ion battery is one type of lithium transition metal oxide that can release oxygen from the crystal or irreversibility transfer phase, at elevated temperature [1,2]. In this respect, numerous studies have been carried out, in order to find a safe and stable cathode material, and also to try to improve the battery safety, and reduce its cost.
Among the known cathode materials, the LiFePO4 ordered olivine-type structure has been attractive, mainly due to its high theoretical specific capacity (~170 mAh/g) [3-5], high stability, low cost, and high compatibility with the environment [6,7]. But its low electronic conductivity (~10-9 S/cm) [8] effect on initial capacity loss, poor rate capacity, and very slow diffusion of Li+ , lead to limitations in its practical application. Many researchers have tried to solve these problems as follows: (1) coating with a conductive layer around the particles [9], (2) substituting the ionic in the materials [10], and (3) changing the particle properties, such as shape, size, texture and phase distribution [11]. LiFePO4 can be synthesized by various methods, such as the solgel method [12], mechanical alloying [13], microwave processing [14], solid-state reaction method [3], emulsion-drying method [9], co-precipitation method [15], and hydrothermal method [16]. Compared with other methods, the hydrothermal synthesis has some advantages, such as simple synthesis process and low energy consumption. Ball-milling (using a planetary mono mill) is also a useful method for synthesizing cathode materials. Due to the very fine nature of the nanoparticles and their large specific surface area that is obtained by this method, the electronic conductivity of LiFePO4-PSS composites can be improved.
Conducting polymers (e.g. polythiophene [17], polypyrrole (PPy) [18], polyaniline (PANI) [20], etc.) have been deeply researched, in order to improve the electronic conductivity of LiFePO4, due to important properties of its: (1) high conductivity in the doped state, and (2) fast charge/discharge electrontransfer kinetics. In this work, we used PSS. PSS is an ionomer (a type of polymer) of polystyrene. Ionomers have unique physical properties, including electrical conductivity and isoviscosity - specifically, increase in ionomer solution viscosity with increasing temperature [19].
In this study, LiFePO4-PSS composites were prepared by a hydrothermal method, and followed by ball-milling. Different PSS were added to improve the electronic conductivity of LiFePO4 nanoparticles. LiFePO4-PSS/Li batteries were fabricated in an argon-filled glove box, and their electrochemical properties were analysed by scanning electron microscopy (SEM), X-ray diffraction (XRD), cyclic voltammetry (CV), and charge/discharge experiments.
LiFePO4-PSS composites were prepared from starting materials of LiOH.H2O, FeSO4.7H20, H3PO4, and C6H8O6. After LiOH.H2O was dissolved in 30 ml distilled water, H3PO4 and FeSO4 powder were added to LiOH solution in a molar ratio of Li: Fe: P= 3:1:1. Addition of C6H8O6 as a reducing agent was useful in preventing the conversion of Fe2+ to Fe3+ and generation of α -Fe2O3 during the heat treatment process. The mixture solution was filled in a Teflon obturation vessel (TAF-SR-50, TAIATSU TECHNO), and was sealed in a stainless steel autoclave, then heated at 170℃ for 12 h. After cooling down to ambient temperature, the solutions were filtered, to separate the precipitate powders. The obtained powders were dried at 110℃ for 1h under vacuum. Then, different wt% PSS (0%, 2.91%, 4.75%, 7.36%) were added to LiFePO4, and milled at 300 rpm for 10 h. The obtained powders were pelleted, and further heated at 500℃ for 1h in a tubular oven, under dry argon flow. After cooling down to room temperature, the LiFePO4-PSS composites were ball-milled again for 10h. Finally, the mixture was dried at 90℃ for 12 h.
The X-ray diffraction (XRD) measurement was carried out on a high resolution X-ray Diffractometer using a Cu Kα radiation source (λ = 1.5406 A ), with a step size of 4º min-1 from 10º to 50º . The morphology of LiFePO4-PSS cathode was characterized by Cold Field Emission scanning electron microscope (FE-SEM).
The composite electrodes were made from mixtures of as-prepared LiFePO4-PSS with SP-270, polyvinylidenefluoride (PVDF) in a weighting ratio of 70%: 25%: 5%. After being ball-millled, the slurry was coated onto aluminum foil, and dried at 90℃ for 1 h before roll-pressing. Then the electrodes were cut into 2× 2 cm2 sections and dried again at 110℃ for 24 h under vacuum. The beaker-type batteries were assembled in an argon-filled glove box, using lithium as the anode, and 1 M LiPF6-EC/DMC (1:1) as the electrolyte. The electrochemical properties of LiFePO4- PSS/ Li batteries were analyzed by charge/discharge tests (WBCS3000, WonATech) in the potential range of 2.0-4.5 V at room temperature and cyclic voltammetry, with scan rate of 0.1 mV s-1 from 2.3 to 4.5 V. To investigate the interface properties and resistance of cell when charging/discharging, the electrochemical impedance was measured, with frequencies ranging from 10 mHz to 2 MHz.
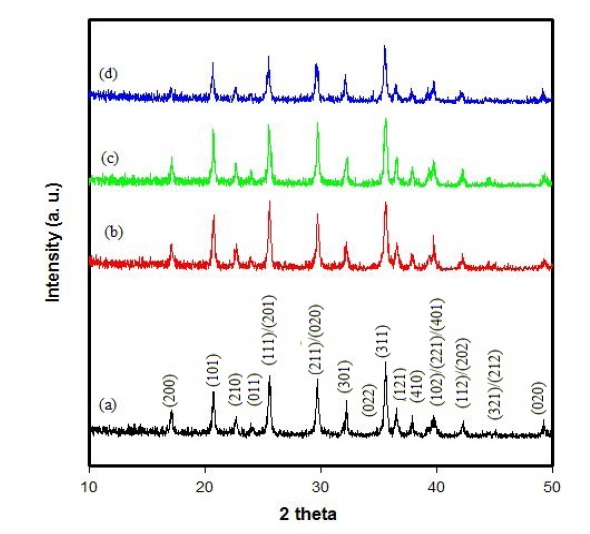
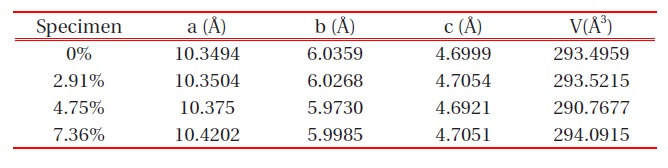
The XRD patterns of LiFePO4-PSS nanoparticles with different wt% PSS conductive additives are shown in Fig. 1. All the patterns can be indexed to a single-phase material, having an orthorhombic olivine-type structure with a space group of Pnma. This is the same as the X-ray powder diffraction data file (JCPDS card number 81-1173) by the American Society for Testing Materials that we call standard. The unit cell parameters for the LiFePO4 sample are: a = 10.3494 A , b = 6.0359 A , c = 4.6999 A and V = 293.5949 A3. The unit cell parameters of the other samples can be seen in Table 1 below, with the best value of LiFePO4 being 4.75 wt% PSS. Its unit cell parameter is a = 10.375 A , b = 5.9730 A , c = 4.6921 A , and the unit cell V = 290.7677 A3. The XRD results dem-
[Table 1.] The unit cell parameters of LiFePO4-PSS composites.
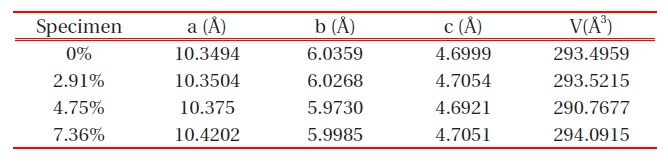
The unit cell parameters of LiFePO4-PSS composites.
onstrate that the only phase observed is LiFePO4. No impurity was found in the LiFePO4-PSS powders. This demonstrates that the PSS-added samples do not change the crystal structure of LiFePO4 nanoparticles.
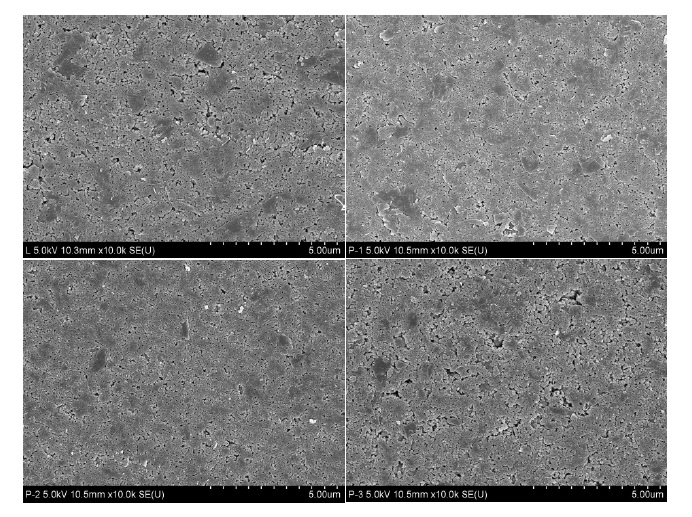
The images of LiFePO4-PSS electrodes are shown in Fig. 2. LiFePO4-PSS electrodes consist of LiFePO4-PSS powders, PVdF, and SP-270. In Fig. 2(a), we can recognize the existence of LiFe- PO4, with conductive material SP-270, and binder PVDF. When making film with conductive material PVdF and binder SP-270, many pores appear. As more pores appear, the movement of electrons is resisted; and as the pore size increases, the chargedischarge efficiency decreases. Since PSS is added, the pore appearance is smaller, and they get clogged. This means that PSS is stuck on the surface of LiFePO4, and forms a layer around the
surface of the LiFePO4 particles. This is helpful to increase reversibility and electronic conductivity of LiFePO4. To prove these assumptions, the electronic conductivities of LiFePO4-PSS electrodes are measured. The results confirm that the electronic conductivity of LiFePO4-PSS electrodes are improved from 1.09× 10-9 S/cm of pure LiFePO4 to 2.75× 10-4 S/cm, 2.47× 10-3 S/cm, 4.32× 10-3 S/cm of LiFePO4 with 2.91 wt%, 4.75 wt%, 7.36 wt% PSS, respectively.
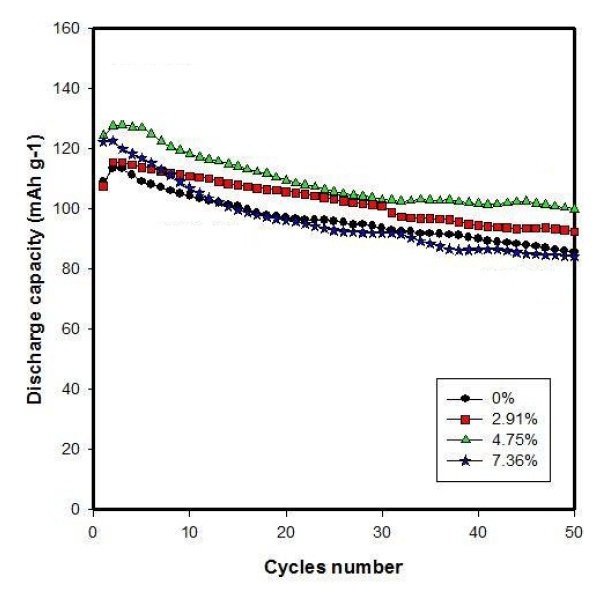
Figure 3 shows the cycling performances of Li/LiFePO4-PSS batteries. As shown in Fig. 3, the Li/LiFePO4-PSS batteries with 2.91 wt%, 4.75 wt%, 7.36 wt% PSS show cycling performance at 2nd cycle with discharge capacities of 115.4 mAh/g, 128 mAh/ g, 122.5 mAh/g; and discharge capacities of 92.4 mAh/g, 100.39 mAh/g, 84.4 mAh/g, respectively, after 50 cycles. In the meantime, the discharge capacity of pure Li /LiFePO4 battery at 2nd cycle is 113.48 mAh/g, and at 50th cycle is 86 mAh/g. The discharge capacity of LiFePO4 with 4.75 wt% PSS showed the best discharge capacity. This was considerably increased, due to the increase in electronic conductivity and specific surface area, and decrease in average crystal size when adding different PSS contents.
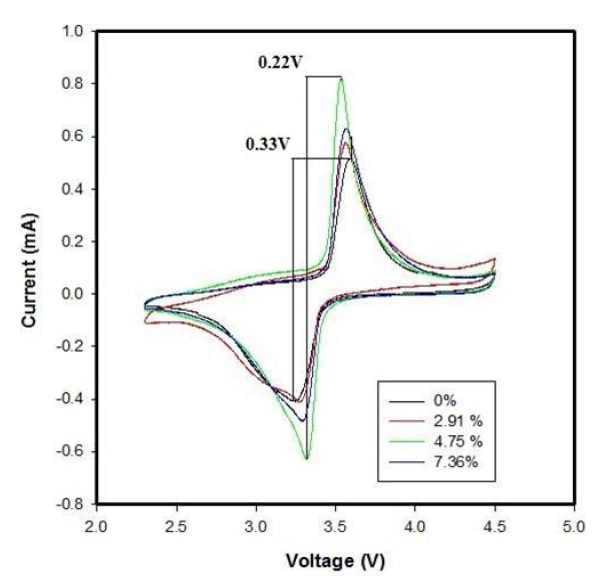
The cyclic voltammograms of LiFePO4-PSS/Li batteries with different PSS conductive additives at 3rd cycle are shown in Fig. 4. As for CV, the voltage difference between oxidation peak and reduction peak is an important parameter in valuing the electrochemical reaction reversibility [20].
As shown in Fig. 4, the oxidation and reduction peaks in the 3rd cycle appear at around 3.59 and 3.24 V, respectively. The voltage difference between the two peaks is 0.35 V. However, in the case of Li/LiFePO4-PSS with 4.75 wt% PSS, the oxidation and reduction peaks in the 3rd cycle appear at around 3.54 and 3.32 V, respectively. The voltage difference between the two peaks is 0.22 V. Meanwhile, the redox peak profiles of Li/LiFePO4-PSS batteries with 4.75 wt% PSS are more symmetric than that of the LiFePO4/ Li battery. This demonstrates that the reversibility and reactivity of Li/LiFePO4-PSS batteries are better than that of LiFePO4/Li batteries.
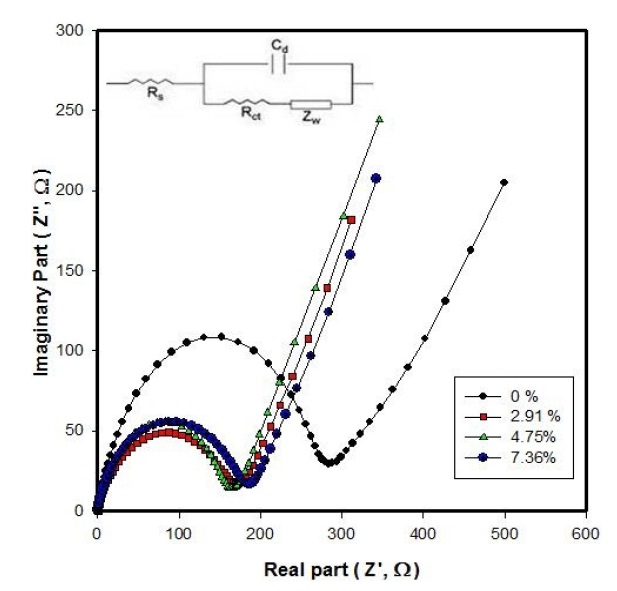
The impedance spectra of Li/LiFePO4-PSS batteries with different wt% PSS after 3 cycles are shown in Fig. 5. As can be seen from Fig. 5, the resistance of Li/LiFePO4-PSS batteries with 0 wt%, 2.91wt%, 4.75 wt%, and 7.36 wt% is 283 Ω , 155 Ω , 150 Ω , 175 Ω , respectively. This is consistent with the results of Fig. 4. This phenomenon can be explained in terms of the electric polarization due to an increase in the IR drop, where I is the current
passing the battery and R is the battery impedance.
LiFePO4-PSS composites have been synthesized successfully, by a hydrothermal method, and subsequent ball-milling. To improve the low electronic conductivity of LiFePO4, different PSS additives were added. The XRD results demonstrate that LiFe- PO4-PSS composites have an orthorhombic olivine-type structure, with a space group of Pnma. A Li/LiFePO4-PSS battery with 4.75 wt% PSS shows the best electrochemical properties, with the highest discharge capacity of 128 mAh/g, and the smallest voltage between the reduction peak and oxidation peak of 0.22 V, the smallest resistance of 150 Ω , and high electronic conductivity of 2.47× 10-3 S/cm.