Many kinds of aluminosilicate minerals, such as zeolite, bentonite, kaoline, mica, etc., have been used as inorganic fillers for conventional polymer composites, in which they not only reduce the cost but also provide special properties, such as electrical insulation, modulus, hardness, thermal stability, and thickening [1-4].These days, multilayered silicates have been introduced to polymer matrixes at the nano-sized order, and the most wellknown are montmorillonite, saponite, and hectorite [5-7]. They are generally introduced into a polymer matrix in a bulk shape with micro-size, and they should be separated into sheet-like monolayers, in order to act as nano particles in a polymer matrix, whose dimensions become (20~1,000)× (20~1,000)×1 nm3. The driving force separating them from each other is given by the penetration characteristics of polymer chains, through the intergalleries between the silicate monolayers, resulting in intercalated or exfoliated nonocomposites [5,6].
In recent years, many researchers have reported on the improvement of electrical properties of an epoxy matrix by the incorporation of nano-sized fillers [8-10]. Our previous work [8] showed that epoxy nanocomposites were remarkably improved by the addition of intercalated silicates. Tanaka and co-workers [9] showed that in needle-plate electrode geometry, epoxy/ layered silicate nanocomposite had much better insulation breakdown time than epoxy resin without fillers. Zou et al [10] reported the nfluence of humidity on the dielectric properties of epoxy nanocomposites filled with silica.
In this study, an epoxy/organoclay nanocomposite was synthesized by in-situ polymerization process, in order to apply the nanocomposite to an electrical insulation material, and the cure kinetics were studied by introducing the differential scanning calorimetry (DSC) data to the Kissinger equation. The thermal degradation kinetics were investigated by thermogravimetric analyzer (TGA) and the Ozawa equation.
Unmodified montmorillonite (MMT) was obtained by purifi-
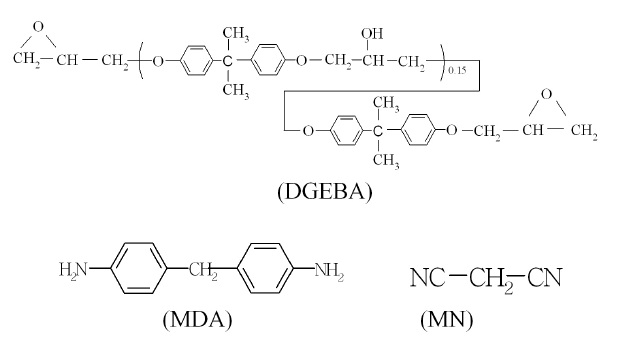
cation of a natural bentonite, mined from the Gampo area, Korea, and was treated with octadecyl triethyl ammonium bromide (ODTMA) [11]. ODTMA was purchased from Aldrich Co. Epoxy matrix consisted of diglycidyl ether of bisphenol A (DGEBA, epoxy base resin), 4,4’-methylene dianiline (MDA, curing agent)), and malononitrile (MN, chain extender). A commercial DGEBA type epoxy resin, whose trade name was YD 128, was purchased from Kukdo Chem. Co., Korea. The epoxy equivalent weight (EEW) was 184~190 g/eq, and the viscosity was 11,500-13,500 cps at 25℃. MDA was obtained from Fluka Chemie AG Co. Its molecular weight MW was 198.3, and melting temperature Tm was 90℃. MN was also purchased from Fluka Chemie AG Co. The molecular structures were as follows.
The modified organoclay (3 phr) was homogenously mixed with epoxy base resin (100 g) for 1 hr using an ultrasonic homogenizer (20 kHz), and the mixture was well mixed with MDA (30 phr) and MN (5 phr) using a mechanical stirrer. The unit phr means the part per one hundred gram of epoxy base resin. Dynamic DSC analysis was performed as follows: about 2~3 mg of the mixed sample was weighed exactly in an aluminum pan, and it was loaded into a DSC furnace (Instrument Specialists Incorporated, DSC Infinity Series, USA), DSC analysis was then performed at the heating rates of 5, 10, 15 and 20℃/min, respectively. Nitrogen flowed at 40 ml/min, to prevent the oxidation of the samples.
The epoxy/organoclay mixture was cured at 150℃ for 1 hr
[Table 1.] Cure kinetic parameters for the epoxy/organoclay (3 phr) system.
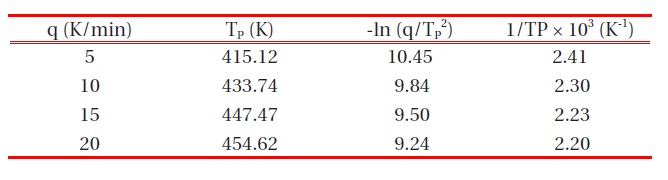
Cure kinetic parameters for the epoxy/organoclay (3 phr) system.
after curing at 80℃ for 1.5 hr. To study thermal degradation of the cured epoxy nanocomposites, a thermogravimetry analyzer (TGA, Cahn-121, USA) was employed at heating rates of 5, 10, 15 and 20℃/min, respectively. Nitrogen flowed at 40 ml/min, to prevent the oxidation of the samples.
The change of the interlayer distance was measured by wideangle X-ray diffractometer (WAXD, XRD30, Rigaku). The X-ray beam was nickel-filtered Cu Kα (λ = 0.154 nm) radiation, operated at a tube voltage of 40 kV, and tube current of 30 mA. The scanning range was 2θ = 2~12o, with a rate of 1o/min.
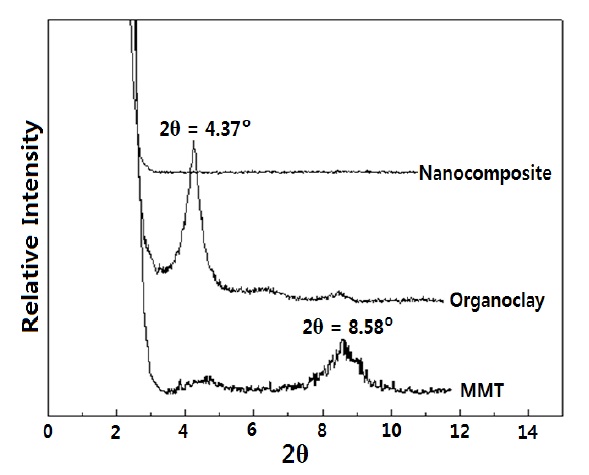
To estimate the formation of nanocomposites, WAXD analysis was carried out for the epoxy nanocomposite, and the unmodified MMT and organoclay were also analyzed by WAXD as references. The patterns are shown in Fig. 2. The characteristic peak for interlayer distance (d-space) of the unmodified MMT appeared at 2θ = 8.58o and was shifted to 2θ = 4.37o in the pattern of the organoclay. The d-space could be calculated by introducing the 2θ values into Bragg’s equation, whose formula was λ =
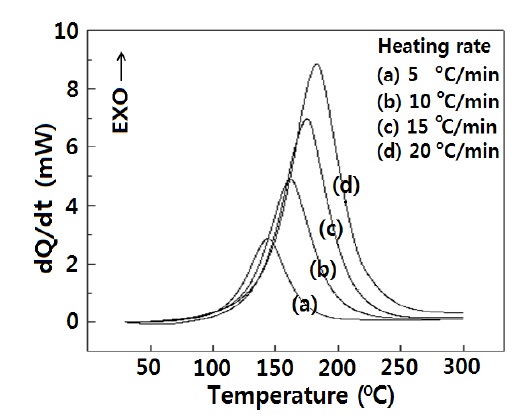
In order to get the cure kinetic parameters, DSC analysis was carried out at different heating rates, and the thermograms are shown in Fig. 3. Each thermogram showed only one exothermic peak, and it is well known that the heat is due to amine-epoxide, epoxy-hydroxyl, amine-nitrile and hydroxyl-nitrile reactions [12].
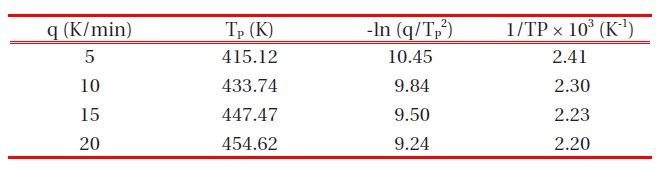
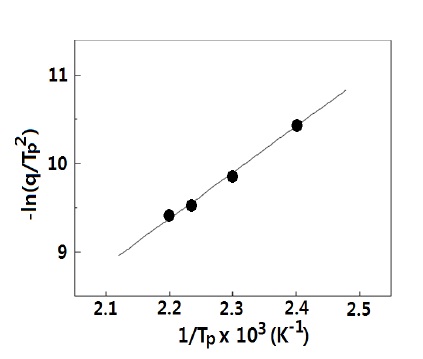
DSC data were modified in the form of -ln(q/TP2) and 1/Tp, in order to introduce them to the Kissinger equation [13], and these values are listed in Table 1.
where, q: heating rate, TP: temperature at a peak, Ea: cure activation energy, A: pre-exponential factor, and R: the universal gas constant, 8.314 J/mol·K.
The activation energy and pre-exponential factor could be calculated by the linear relationship of -ln(q/TP 2) vs. 1/Tp, as shown in Fig. 4. From the slope and y-intersection, the activation energy and pre-exponential factor, respectively, could be obtained. The activation energy for cure kinetics was 43.3 kJ/mol, and the preexponential factor was 2.50 x 106 min-1. The activation energy and pre-exponential factor values of cure kinetics for DGEBA/ MDA (30 phr)/MN (5 phr) without any organoclay were also calculated via the same procedure, and we obtained the activation energy of 45.8 kJ/mol and the pre-exponential factor of 2.58 × 106 min-1. These values impiied that the organoclays had almost no effect on the cure kinetics of the neat epoxy matrix.
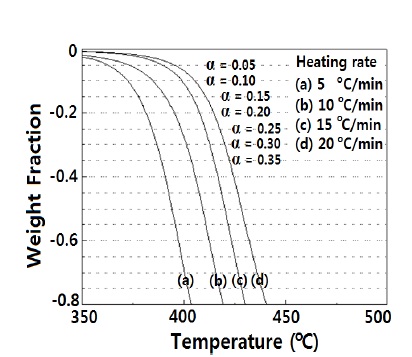
In order to get the thermal degradation activation energy, TGA analysis was carried out at different heating rates, and the data are shown in Fig. 5. The nanocomposite started to degrade at about 350℃, in the thermogram obtained by a heating rate of 5℃ /min.
Ozawa equation [14] was used as follows.

Thermal degradation activation energy for epoxy/organoclay (3 phr) system obtained from Ozawa equation.
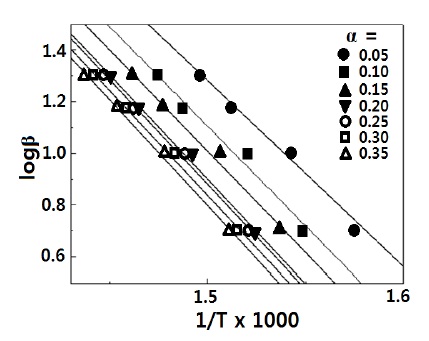
where, β : heating rate, Ed: thermal degradation activation energy, R: the universal gas constant, 8.314 J/molㆍK, and α : a degradation conversion. The straight lines of logβ against 1/T are plotted in Fig. 6, and the activation energies of thermal degradation were determined from the slope, because the slope values were given by 0.4567ㆍEd/R, and are listed in Table 2. The Ed value, 129 kJ/mol at α = 0.05 was clearly distinguished from other Ed values, ca. 145 kJ/mol of α = 0.10~0.35. The near the same values over the range of 0.10~0.35 indicated that the same mechanism was acting on the degradation of the crosslinked chains. However, the lower Ed value at α = 0.05 was affected by another mechanism,because of the volatilization of unreacted molecules or moistures. The activation energy of thermal degradation for DGEBA/MDA (30 phr)/ MN (5 phr) without any organoclay (3 phr) was also calculated, via the same procedure, and it was found that the degradation temperature was appeared at about 345℃ , which was 5℃ lower than the system with 3 phr organoclay.The average activation value was 214.32 kJ/mol, which was 6.25 kJ/mol lower. These results meant that the organoclay could bring some retarding effect on the thermal degradation of the epoxy matrix.
Epoxy nanocomposite was prepared by an in-situ polymerization process, and the exfolication of the organoclay was estimated by WAXD analysis. The WAXD peak at 2θ=4.37o (dspace= 2.02 nm) for organoclay had absolutely disappeared in the cured nanocomposite system, which meant that the silicate layers became disordered during the cure reaction, due to the delamination of the organoclay. The DSC data and Kissinger equation showed that the cure kinetics were 45.8 kJ/mol, and the pre-exponential factor was 2.58 × 106 min-1; and the organoclay had almost no effect on the cure kinetics of the neat epoxy matrix. The TGA data and Ozawa equation showed that the average activation energy for thermal degradation was 143 kJ/mol, and the organoclay could bring some retarding effect on the thermal degradation of the epoxy matrix