Environmental pollution by heavy metals is a serious and complex problem that at all times occupies worldwide attention. Heavy metals are classified as the chief surface and groundwater pollutants. Industrial and municipal wastewaters frequently contain metal ions that can be harmful to aquatic life and human health. Heavy metals are usually found in industrial wastewater, for example, that from the production of textiles, from paint manufacturing, from leather tanning, etc. [1]. In general, heavy metals are not biodegradable and they tend to accumulate in living organisms, causing cause various diseases and disorders. In adults, Pb (II) can increase blood pressure and cause fertility problems, nerve disorders, muscle and joint pain, irritability, and memory or concentration problems [2]. The treatment methods for metal-bearing effluents commonly include chemical precipitation, membrane filtration, electrolytic reduction, solvent extraction, ion exchange, and adsorption [3]. Among these treatments, adsorption is considered to be an effective and economical method and has attracted considerable interest. Activated carbons are adsorbents that are suitable for industrial use in multiple processes for product separation and purification, and for the treatment of liquid and gaseous effluents. Considering the high cost of activated carbon and the tedious procedures for the preparation and regeneration of activated carbons, there is a continuing search for low-cost potential adsorbents. Lignocellulosic materials and wastes such as peanut skins, cotton, onion skins, rice hulls, maize stalks, bark, jute fibers, bagasse, rice straw, corncobs, and palm kernel husks have received much attention in the field of heavy metal ion removal [4]. Various functional groups such as carboxylates, phenolic and aliphatic hydroxyls, and carbonyl groups in these materials have the ability to adsorb some metal ions. In order to increase the metal ion adsorption in cellulose, it was chemically modified by introducing different groups such as phosphoric acid (H3PO4) and potassium carbonate (K2CO3) [5].
2.1.1. Physical activation
Walnut shells were obtained from local natural resources. After they were obtained, the fresh walnut shells were washed several times with distilled water to remove surface impurities; shells were then dried at room temperature for one day. The samples were heated and burned and the charcoal was crushed with a grinder and then ground fine enough for the powder to pass through a 100-mesh sieve for further experiments. Powder was activated to a final temperature of 500℃ by heating it in a muffle furnace for six hours.
2.1.2. Chemical activation
Chemical activation methods using H3PO4 and K2CO3 were used to activate the raw material. One gram of each raw material was weighed and then the weighed raw sample was impregnated in 5 mL of 50% (v/v) concentration of phosphoric acid and 5 mL of 20% (w/v) concentration of potassium carbonate for 1 h at room temperature. After that, the blend was transferred to a muffle furnace, where carbonization was carried out under an air atmosphere. The plant material was chemically modified with H3PO4 and K2CO3 in three steps of temperatures and times. The furnace was heated to 200℃ and maintained at this temperature for 30 min in order to allow the free evolution of water; a black sticky solid was obtained. Then, the furnace was heated to 500℃, and maintained at this temperature for 60 min. finally the furnace was heated to 700℃ and maintained at this temperature for 15 min. After cooling to room temperature, the solid was washed with ultra pure water at 25℃ to remove excess H3PO4 and K2CO3. The carbon samples were dried at 50℃
2.2. Batch equilibrium studies
Activated carbon (0.05 g) was added to the solutions of Pb(NO3)2 with different initial concentrations. The mixture was stirred for 15 min at room temperature (25℃). The amount of remaining metal ions was determined using an atomic absorption spectrophotometer (Shimadzu-Japan). The amount of Pb (II) adsorbed on the adsorbent at adsorption equilibrium was calculated according to the following Eq. (1)
where C0 and Ce (mg/L) are the initial and equilibrium Pb (II) concentrations, respectively. V is the volume of the solution (L) and W is the mass of adsorbent used (g).
In the order to study the effect of contact time between adsorbent and adsorbate, at first, we put 20 ppm of Pb (II) and 0.05 g of activated carbon in contact for 240 min; with this contact of 0.05 g of activated carbon, a maximum of adsorbtion took place in the first 15 min of contact.
To analyze the effect of initial Pb (II) concentration, at first we changed the concentration of Pb (II), in a range from 5 to 23 ppm. Then, we put the samples in contact with the adsorbent for 15 min; at this stage, maximum absorbtion in 20 ppm concentration of Pb (II) had taken place.
The effect of the pH on the metal adsorption for modified activated carbon was studied for pH 3, 4, 5, 6, 7, 8, 9, and 11, at which the material exhibits chemical stability.
The effect of activated carbon from walnut shells (ACW) dosage on the adsorption process was investigated by varying the sorbent dosage from 0.01 g-1 g of Pb (II) solution. The experiment was conducted by measuring 20 ppm of Pb (II) solution. Appropriate dosage of the ACW was measured into the sample and sample was agitated for 15-240 min.
According to the Langmuir model, adsorption occurs uniformly on the active sites of the adsorbent, and once an adsorbate occupies a site, no further adsorption can take place at this site. Thus, the Langmuir model is given by equation [6].
where qmax and b, the Langmuir constants, are the saturated monolayer adsorption capacity and the adsorption equilibrium constant, respectively. A plot of Ce/qe versus Ce would result in a straight line with a slope of (1/qmax) and intercept of 1/ bqmax The Langmuir parameters can be used to predict the affinity between the sorbate and the adsorbent using the dimensionless separation factor RL:
The RL value indicates the shape of the isotherm, as follows [7]:
RL > 1 Unfavorable
RL =1 Linear
0 < RL < 1 Favorable
RL = 0 Irreversible
The Freundlich model stipulates that the ratio of solute adsorbed to the solute concentration is a function of the solution. The empirical model was shown to be consistent with an exponential distribution of active centers, characteristic of heterogeneous surfaces. The amount of Pb (II) adsorbed onto the modified ACW at equilibrium, qe, is related to the concentration of Pb (II) in the solution, Ce, following [8]:
This expression can be linearized to give
where Kf and n are the Freundlich constants, which represent adsorption capacity and adsorption intensity, respectively. A plot of lnqe versus lnCe would result in a straight line with a slope of (1/n) and intercept of lnK f.
The Dubinin-Radushkevich (D-R) isotherm was employed in the following linear form [9]:
The Polanyi potential ε, can be expressed as
The Temkin equation suggests a linear decrease of sorption energy as the degree of completion of the sorptional centers of an adsorbent is increased. The Temkin isotherm has been generally applied in the following form [10,11]:
where B=RT/b, R is the gas constant, b is the Temkin isotherm constant, T is the absolute temperature (K), and A is the Temkin isotherm constant (L/mg), Therefore, by plotting qe versus ln Ce, one can determine the constants A and B.
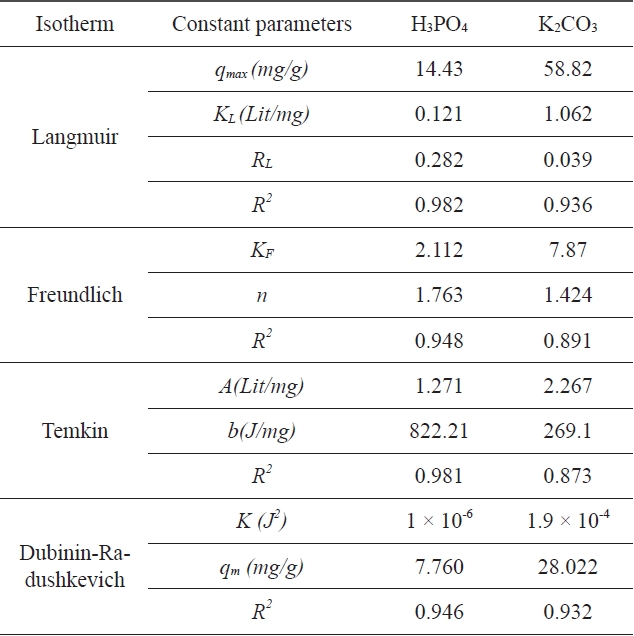
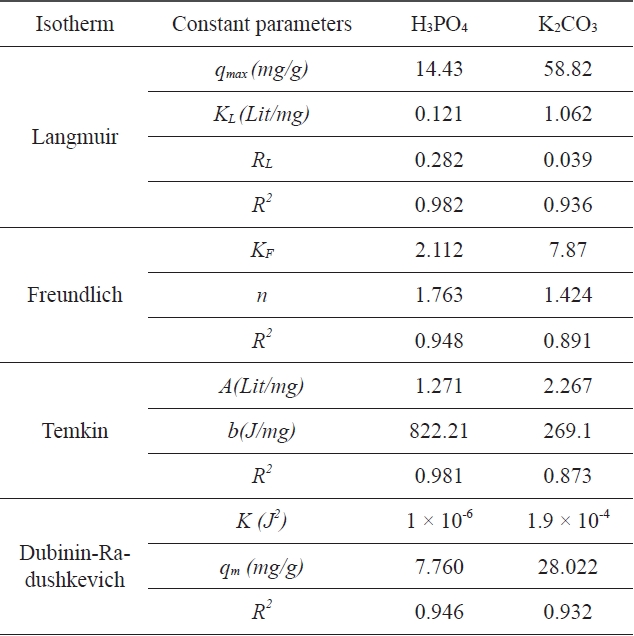
A list of the parameters obtained, together with the R2 values, is given in Table 1. The experimental results were analyzed by using Langmuir, Freundlich, Tempkin, and D-R isotherm models. The correlation coefficients for the Langmuir and Tempkin equations fitted better than those of the Freundlich and D-R equations for activated carbon modified by H3PO4; the correlation coefficients for the Langmuir and D-R equations fitted better than those of the Freundlich and Tempkin equations for activated carbon modified by K2CO3. The experimental data indicate that the adsorption isotherms are well described by the
Comparison of the coefficients isotherm parameters for adsorption of Pb (II) onto activated carbon modified by H3PO4 and K2CO3
Langmuir isotherm equation and that the calculated adsorption capacity of activated carbon was 14.43 (mg/g) at 25℃ for activated carbon modified by H3PO4 and 58.82 (mg/g) for activated carbon modified by K2CO3. Based on the data in Table 1, it is clear that the correlation coefficient R2 L is comparatively higher than R2 F, R2 T , and R2 (D-R). The value of RL for the adsorption of Pb (II) with activated carbon modified by H3PO4 was 0.282 and the value of RL for the adsorption of Pb (II) with activated carbon modified by K2CO3 was 0.039. These values indicate that the adsorption behavior of activated carbon was favorable.
In order to elucidate the mechanisms and the possible rates of solute adsorption of solutions, different kinetic models have been applied.
The pseudo first-order equation was presented by Lagergren [9] for the sorption of oxalic acid and malonic acid onto charcoal. Afterwards, many researchers reported first order Lagergern kinetics for adsorption of different pollutants on different sorbents [13]. The linearized form of the pseudo first-order equation of Lagergren is generally expressed as follows [14]:
where qt is the amount of Pb (II) adsorbed (mg/g) at time t, k1 is the equilibrium rate constant of the pseudo-first order kinetics (min-1), and t is the constant time (min). According to Eq. (9), the plots of
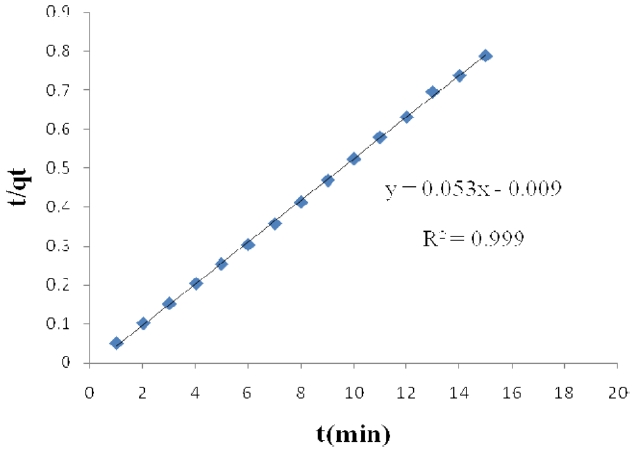
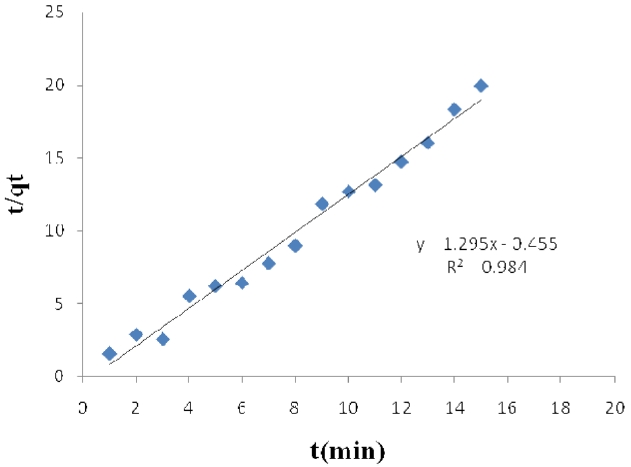
The linearized form of the pseudo- second order chemisorptions kinetic rate equation is presented below [15-18]:
where k2 (g/mg min) is the rate constant for the pseudo- second order adsorption. The intercept and slope of
vs. t (Figs. 1 and 2) were used to calculate the pseudo- second order rate constants k2 and qe, respectively.
The results obtained from these studies were analyzed by using pseudo- first order and pseudo- second order kinetic models. The calculated qe values agree very well with the experimental data; also, the correlation coefficients for the second-order kinetic model are higher than 0.99 in all cases for activated carbon modified by K2CO3 and H3PO4. These results indicate that the adsorption of acid dyes from wastewater onto activated carbon obeys the pseudo- second order kinetic model.
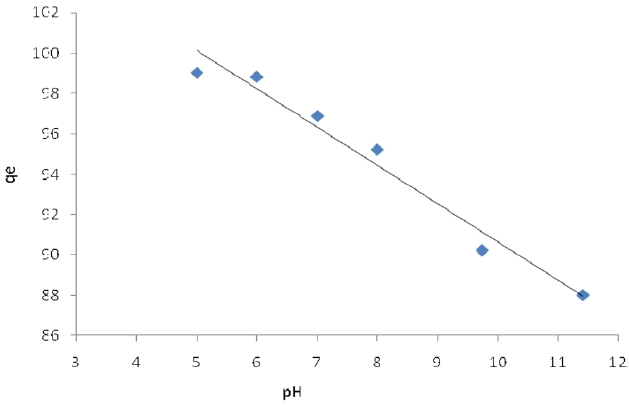
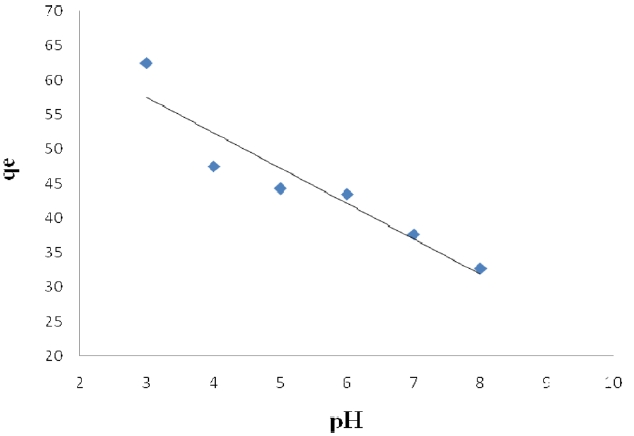
The pH level of the aqueous solution is an important variable for the adsorption of metals onto the adsorbent. The effect of the pH on Pb (II) adsorption by activated carbon modified was studied for pH 3, 4, 5, 6, 7, 8, 9, and 11, at which the material exhibits chemical stability. The efficiency of Pb (II) sorption depends on the pH level of the aqueous solutions. The various influences of pH on the adsorption of Pb (II) onto modified activated carbon are shown in Figs. 3 and 4. It is apparent that using the solution at pH>1 gives the highest removal of Pb (II) on activated carbon modified by K2CO3. We can see that the adsorption capacity is low at pH<1 and that Pb (II) adsorption decreases with the pH decrease from 4 to 8
Pb (II) on activated carbon modified by K2CO3. The amount of adsorbed Pb (II) is at its minimum rate at pH > 6 on activated carbon modified by H3PO4; the maximum adsorption capacities were calculated at pH = 5.
The effect of contact time on the amount of Pb (II) adsorbed per gram of ACW by H3PO4 and K2CO3 was investigated. Equilibrium is reached after 15 min of immersion; the adsorption process is a relatively fast one.
The maximum adsorption, mentioned above, is due to the following factors:
1) Reduction of the weight of activated carbon
2) Reduction in the speed of pore development
3) Filling of pores during the activation time
3.5. Effect of initial Pb (II) concentration
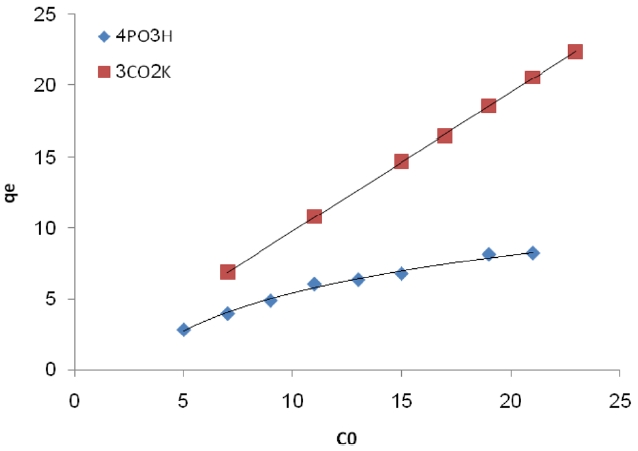
The effect of initial dye concentration has been studied and results are presented in Fig. 5. These results show the effect of the Pb (II) concentration on the adsorption of the different levels of Pb (II) at pH 5. The figure shows that the amount of Pb
(II) adsorbed increases with increasing concentration and then tends to level off. The maximum milligrams per gram of Pb (II) adsorption is 8.23.
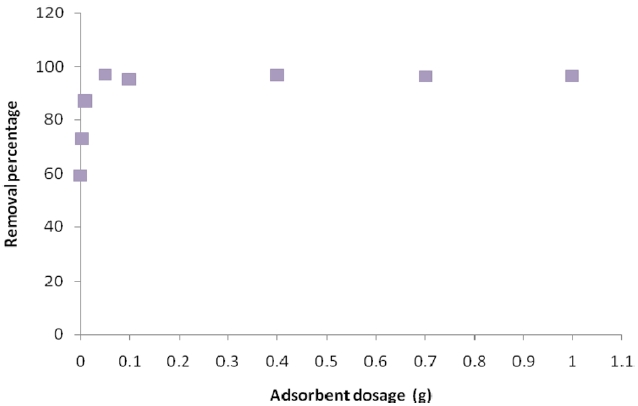
Fig. 6 shows the effect of ACW according to H3PO4 and K2CO3 dose on the removal percentage of Pb (II). The effect of ACW dosage on the adsorption process was investigated by varying the sorbent dosage from 0.01-1 g of Pb (II) solution. The experiment was conducted by measuring 20 ppm of Pb (II) solution. The appropriate dosage of ACW was measured in and sample was agitated for 15-240 min. Samples were withdrawn at fixed intervals and analyzed for residual Pb (II); the amount of sorbate sorbed per unit mass of the ACW was calculated by using the mass balance procedure.
Activated carbon adsorption of metallic ions (mainly Pb (II)), at levels similar to or even better than those obtained using commercial products, was performed by chemical activation of walnut shells.
The results show that as the amount of the adsorbent increased, the percentage of Pb (II) removal increased accordingly.
The optimum pH value for lead adsorption was determined to be 5. The maximum removal of Pb (II) was obtained at pH 5, at a level of 98.84% for activated carbon modified by H3PO4 and 99.03% for activated carbon modified by K2CO3; the adsorbent dose was 0.05 g and the initial Pb (II) concentration at room temperature was 20 mg/L .
The experimental results were analyzed using Langmuir, Freundlich, Tempkin and D-R isotherm models. The correlation coefficients for the Langmuir and Tempkin equations fitted better than those of the Freundlich and D-R equations for the activated carbon modified by H3PO4 and the correlation coefficients for Langmuir and D-R equations fitted better than Freundlich and Tempkin equations for the activated carbon modified by K2CO3.
The results of this investigation show that activated carbon modified by H3PO4 and K2CO3 has a suitable adsorption capacity for the removal of Pb (II) from aqueous solutions.