Recently, superhydrophobic and conductive materials have attracted attention from researchers due to their importance in fundamental research and potential industrial applications in such areas as electronic devices, transparent films, and structural coatings [1-5]. In particular, hydrophobic and conductive films based on multi-walled carbon nanotubes (MWCNTs) have many potential applications due to their novel properties such as high aspect ratio, small radius of curvature, high mechanical strength, thermal/electrical conductivity, and good chemical stability [6-9]. However, for typical carbon nanotubes, polar substances such as water cannot wet the nanotube surface easily [10]. A large number of MWCNT applications are hampered by the hydrophobic properties of these types of CNTs. It is desirable to be able to modify the hydration properties of the surfaces of carbon nanotubes so that they become hydrophilic. The wettability of MWCNTs is one of the most important ideas in both theoretical research and practical applications [11].
Generally, superhydrophobic surfaces have a water contact angle higher than 150° and a water sliding angle of less than 10° [12]. To date, many researchers have endeavored to fabricate superhydrophobic CNTs using aligned-CNTs [11-14]. Huang et al. [13] reported a stable superhydrophobic surface composed of aligned carbon nanotubes (CNTs). However, the area of the aligned-CNT films could be limited due to the requirements of the specific preparation methods. Most recently, several studies have reported a means of preparing superhydrophobic surfaces on non-aligned CNTs [5-9,14-16]. For example, Zou et al. [6] fabricated a superhydrophobic surface (154°~160°) with conductivity using a conjugated block copolymer.
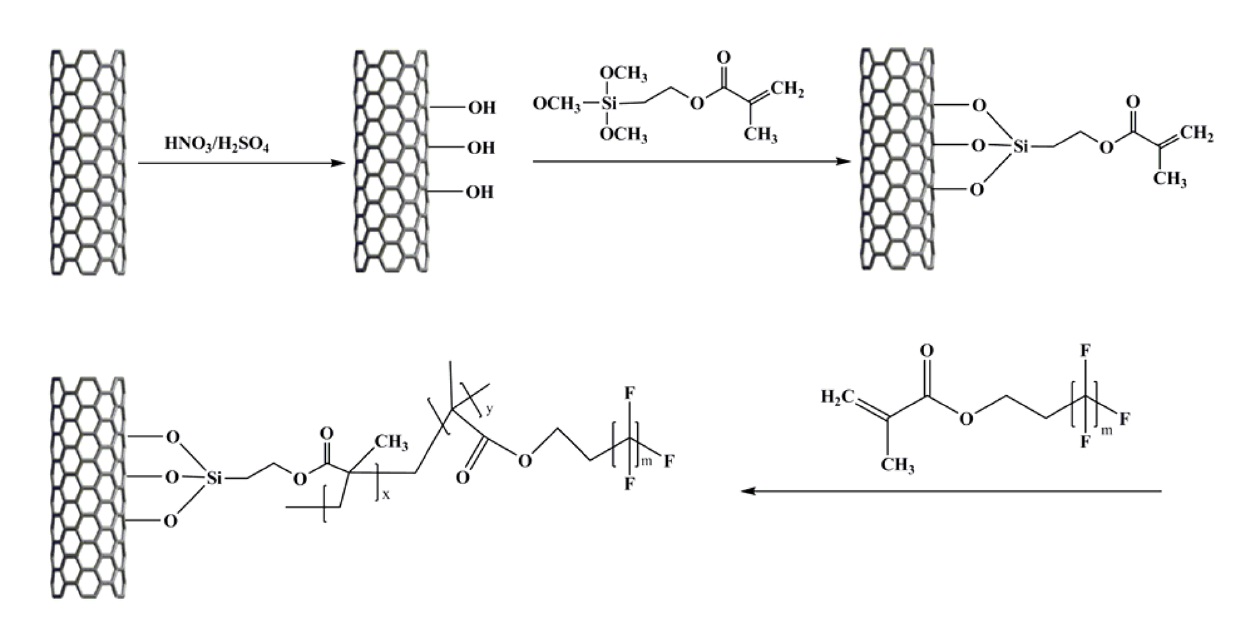
In this work, superhydrophobic coatings of multi-walled carbon nanotubes (MWCNTs) were produced by the radical polymerization of 3-(trimethoxysilyl) propyl methacrylate modified MWCNTs and fluoro acrylate/ methyl methacrylate.
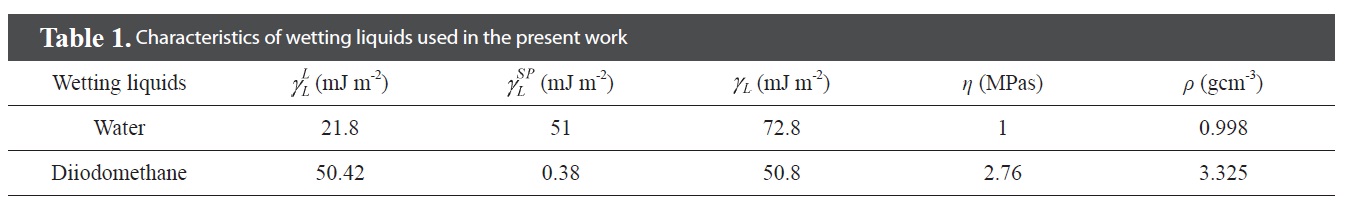
[Table 1.] Characteristics of wetting liquids used in the present work
Characteristics of wetting liquids used in the present work
2.1. Materials and sample preparation
The MWCNTs (Nano Solution Co.) were treated with HNO3/ H2SO4 mixture solutions [17,18]. The oxidized MWCNTs (CNTH) were modified with 3-(trimethoxysilyl) propyl methacrylate (TPM) in toluene at 383 K for 24 h and were then washed thoroughly with toluene several times. The obtained sample is denoted as CNT-Si. Finally, TPM-modified MWCNTs, fluoromonomer (Zonyl® TM fluoromonomer, average Mn of about 534), methyl methacrylate, and AIBN were placed in a vial and dissolved in tetrahydrofuran (THF). The mixture was degassed under a continuous stream of dry argon. A polymerization reaction was carried out at 70℃ for 24 h and this was followed by washing with THF. This sample is denoted as CNT-F (Fig. 1).
X-ray photoelectron spectroscopy (XPS) measurements were done on an ESCALAB220i-XL (VG Scientific, UK) spectrometer with monochromatized Al Kα X-ray radiation as the X-ray source for excitation to confirm the surface functional groups of the treated MWCNTs. Fourier transform infrared (FT-IR) spectroscopy on KBr pellets was performed with a Bio-Rad Co. Digilab FTS-165. Corresponding images of the resulting films were obtained by scanning electron microscopy (SEM, HITA-CHI S4200). The contact angle (CA) of the film was determined to be 20 ± 1°C using the sessile drop method (Phoenix Series Contact Angle Analyzer, Phoenix 150/300). Distilled water and diiodomethane were selected for measurement of the contact angle of the films. For each sample, each calculated contact angle was an average of 10 measurements with a standard deviation below 1° [19]. The surface free energy and other characteristics of the three wetting liquids are listed in Table 1.
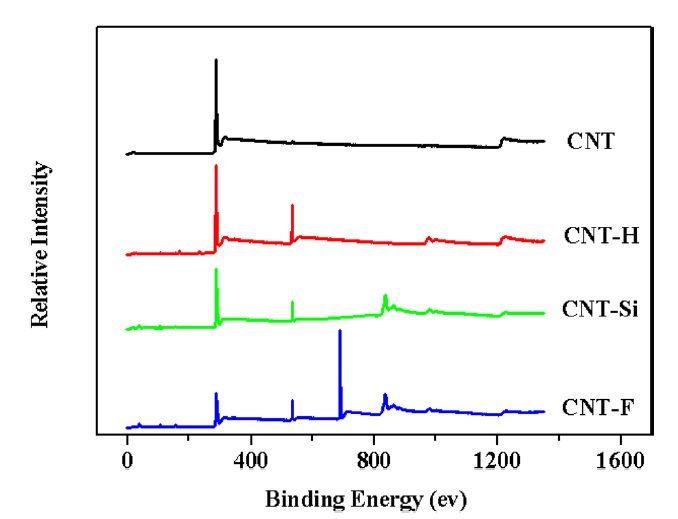
XPS was used to investigate the introduction of elements on the MWCNT surfaces, as shown in Fig. 2. In the figure, the XPS spectrum of the samples shows carbon peaks (285.08 ev), oxygen peaks (532.08 ev), silicon peaks (835.07 ev), and fluorine peaks (690.08 ev). The XPS spectrum of CNTs-H shows oxygen peaks (533.4) that are attributable to an increase in the number of surface oxygen groups (C-O, O=C-O), which are the result of chemical oxidation [17]. The silicon peak (835.07 ev) and the fluorine peak (690.08 ev) are attributable to silanol groups and fluorine groups (-CF-), respectively [20]. This suggests that the fluoromonomer is grafted onto the surfaces of the CNT-Si via chemical modification.
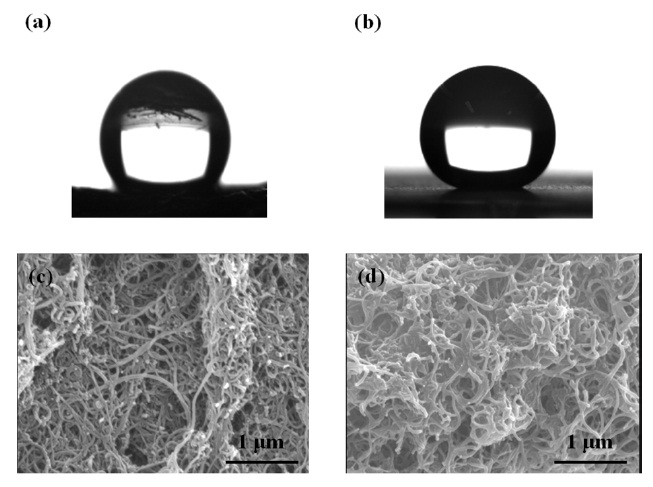
Figs. 3a and b shows schematic diagrams illustrating the changes of the contact angle and the surface properties. As shown in Fig. 3a, the water droplet retains an ellipsoidal shape on the MWCNTs; the droplet has a contact angle of 115.5°, sug-
gesting that the pristine MWCNTs are hydrophobic [16]. The CA of CNT-F increases to 157.7° after it is polymerized with fluoromonomer because the surface of CNT-Si is grafted with ? CF? groups (Fig. 3b). This indicates that the wettability of MWCNTs can become superhydrophobic by varying the chemical composition of the MWCNTs [5]. According to Liu et al. [10], the wettability of CNTs is controlled by the surface energy and surface roughness. The surface roughness of the MWCNTs and CNT-F were examined by SEM, with resulting images shown in Figs. 3c and d. The pristine MWCNT films showed an assembled bundle structure (Fig. 3c). After polymerization, the images revealed that the surface roughness of the MWCNTs contained porous MWCNT networks. The porous CNT-F networks became deeper and larger than those of the pristine MWCNTs, leading to the formation of a hierarchical 3D interpenetrating network. The surface roughness allowed air to become trapped between the solid surface and the water droplet, which decreased the contact
area with water. According to previous research, trapped air is thought to be an important factor that affects the degree of superhydrophobicity [5,14-16]. Therefore, these results show that the hydrophobic surfaces of films are affected by the -C-F- groups and by the surface roughness.
To obtain more detailed information pertaining to the surface energetics of the CNT-F films, the surface free energy was analyzed based on the method of Fowkes [21]. The total surface free energy can be divided into the London dispersive and specific (or polar) components.
where
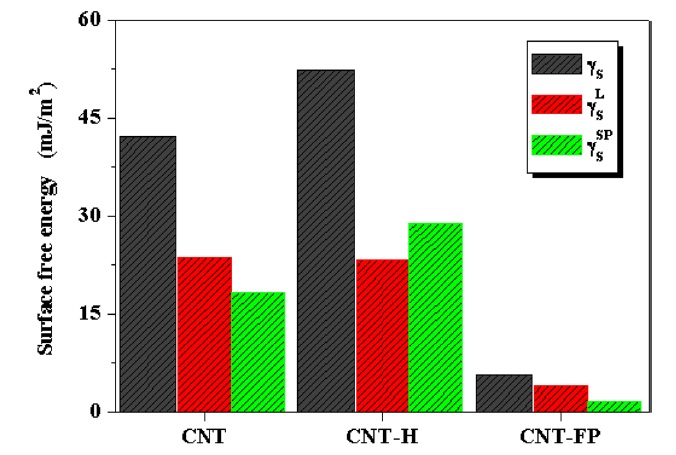
where θ is the contact angle of a liquid droplet in the solid state. The surface free energy components of the samples are shown in Fig. 4. As reported in our previous work, the specific component,
As shown in Fig. 4, the total surface energy and
In conclusion, we successfully prepared superhydrophobic films based on fluoromonomer-grafted MWCNTs. The surface of the films exhibited a high contact angle of 157.7°. The 3D MWCNT networks and the low surface energy of the -CFgroups play important roles in creating the superhydrophobic surface of the MWCNT films. The stable superhydrophobic MWCNT films proposed in this study can be used in many industries and can be extended to control the adhesion properties of a wide variety of superhydrophobic functional materials.