The primary causative agent of ciguatera fish poison-ing (CFP) are nonplanktonic dinoflagellates of the genus
Species level description are very difficult for anteri-posterior compressed dinoflagellate cells such as
The first case of CFP in Indian Ocean was described in Mauritius, an island in the western Indian Ocean (Quod and Turquet 1996). This is first observation of the Cigutara species from the northern part of the Indian Ocean, Paki-
stan is described according to modern taxonomical clas-sification following Faust (1995), Holmes (1998), Chinain et al. (1999), Litaker et al. (2009). This study provides basic information about the toxic benthic dinoflagellates from the non-coral area of Manora Channel with high man-grove vegetation along the Karachi coast and the Coral reef area of the Balochistan coast Pakistan.
Samples sites were the Manora Channel Sindh coast of Karachi and Churna Island Balochistan coast. The Sindh coastline is about 250 km long and the continental shelf deep stretches into the ocean and adjacent to souther-eastern part to Indian border along Sir Creek on the east. The Balochistan coast is 800 km long with a steep and narrow continental shelf to the Iranian border near Jiwani in the west. These coastal areas are commercially impor-tant and provide a transit trade port via Karachi harbor and Gawader port of Balochistan.
Samples were collected from Manora Channel (24°47.93? N, 66°58.87? E) Sindh coast and Churna Island (24°46.70? N, 66°26.66?40˝ E) Balochistan coast of Pakistan (Fig. 1). The samples from the Manora channel were col-lected with a 55 μm mesh size net during July 2007 and a Niskin bottle (1.7 L) sampler at 1 m depth from the Chur-na Island, Balochistan coast during November 2007. The water temperatures from both locations were 32℃ and 28℃, respectively.
Samples were preserved in Lugols solution and brought to the Marine Biotoxin lab, USA and examined under 5600LV Scanning electron microscopy (JOEL, Tokyo, Ja-pan) and under light microscopy / Fluorescence micros-copy (BX51; Olympus, Tokyo, Japan) with a DP71 camera. Cells were stained with calcoflour white, examined under the 200-400× and images were captured with camera. For scanning electron micrograph, Lugol’s fixed sample were desalted in seawater gradient (100, 70, 50, and 25%) and cleaned with 100% freshwater, dehydrated in an acetone series (10, 30, 60, 80, and 100%), mounted and sputter coated with gold-platinum (Desk IV; Denton Vacuum, Moorestown, NJ, USA). The morphometric analysis was performed using Microsuite 5 (Olympus).
Five species of
>
Gambierdiscus toxicus Adachi and Fukuyo 1979 (
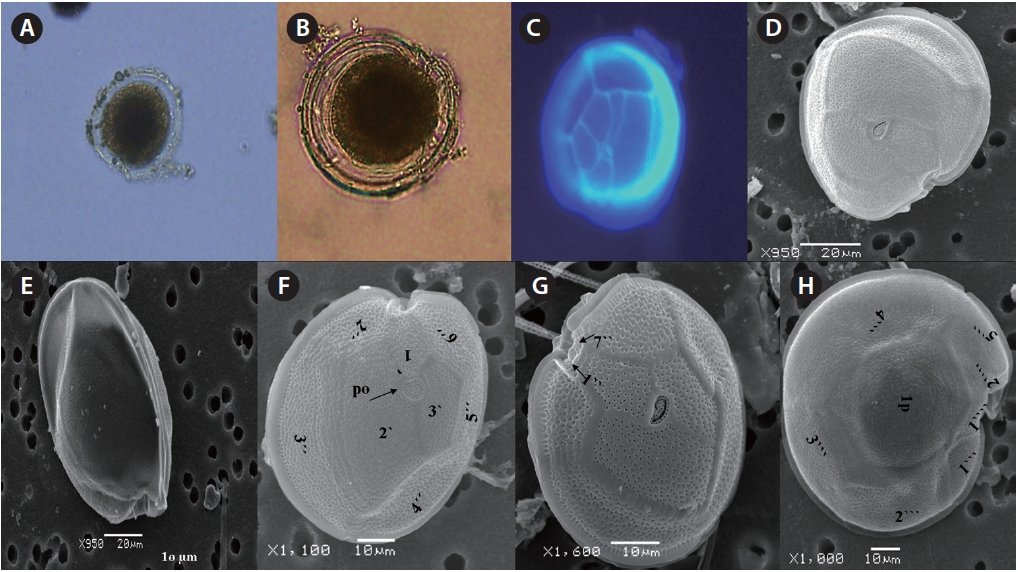
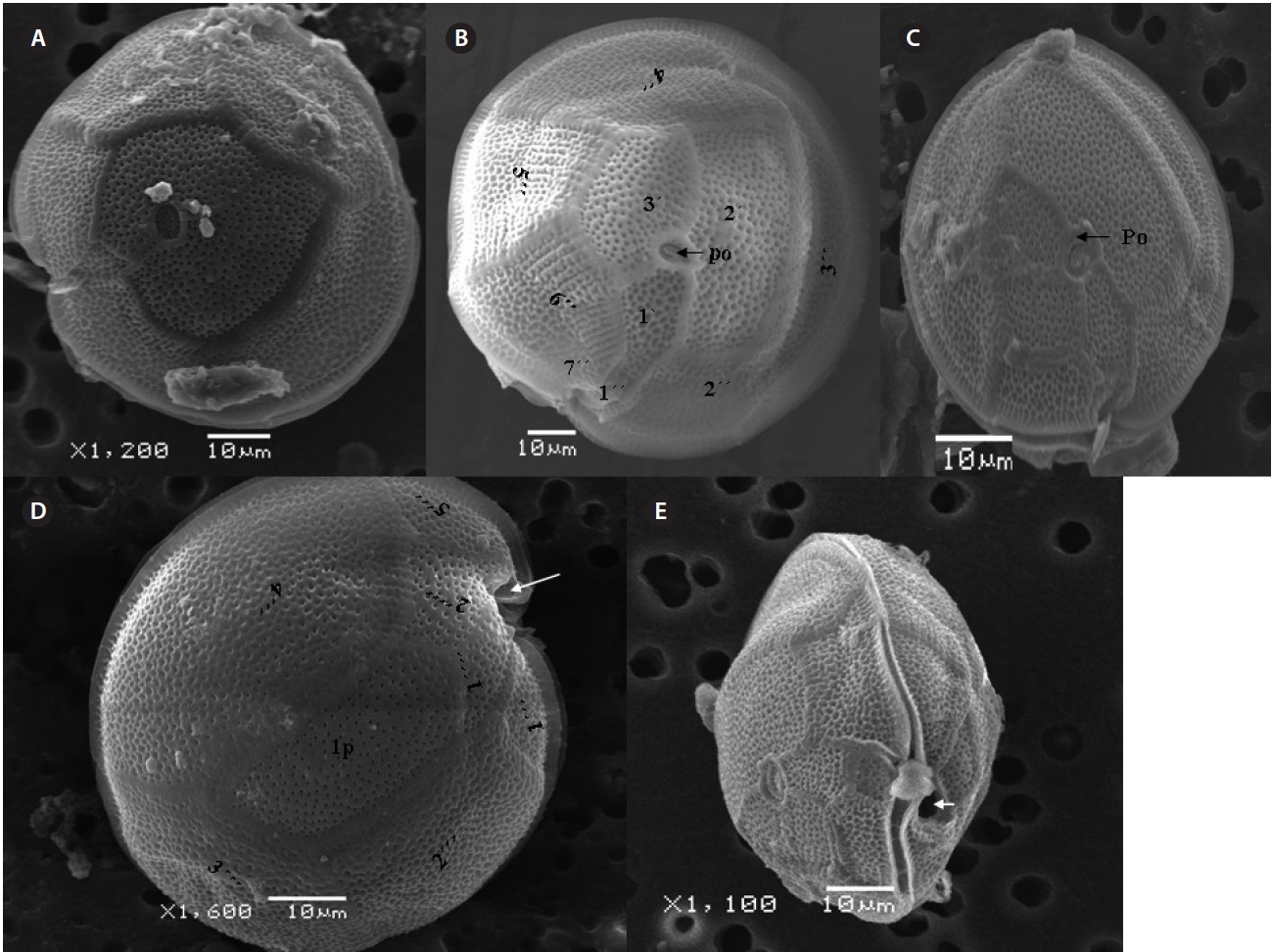
Cells of
μm wide, comes in contact with 2˝, 3˝, and 4˝ plates. Of the precingular plates, 1˝ and 7˝ are smallest, and 3˝ much larger 51.3-68.8 μm and 8.0-14.5 μm in wide (Fig. 2F & G).
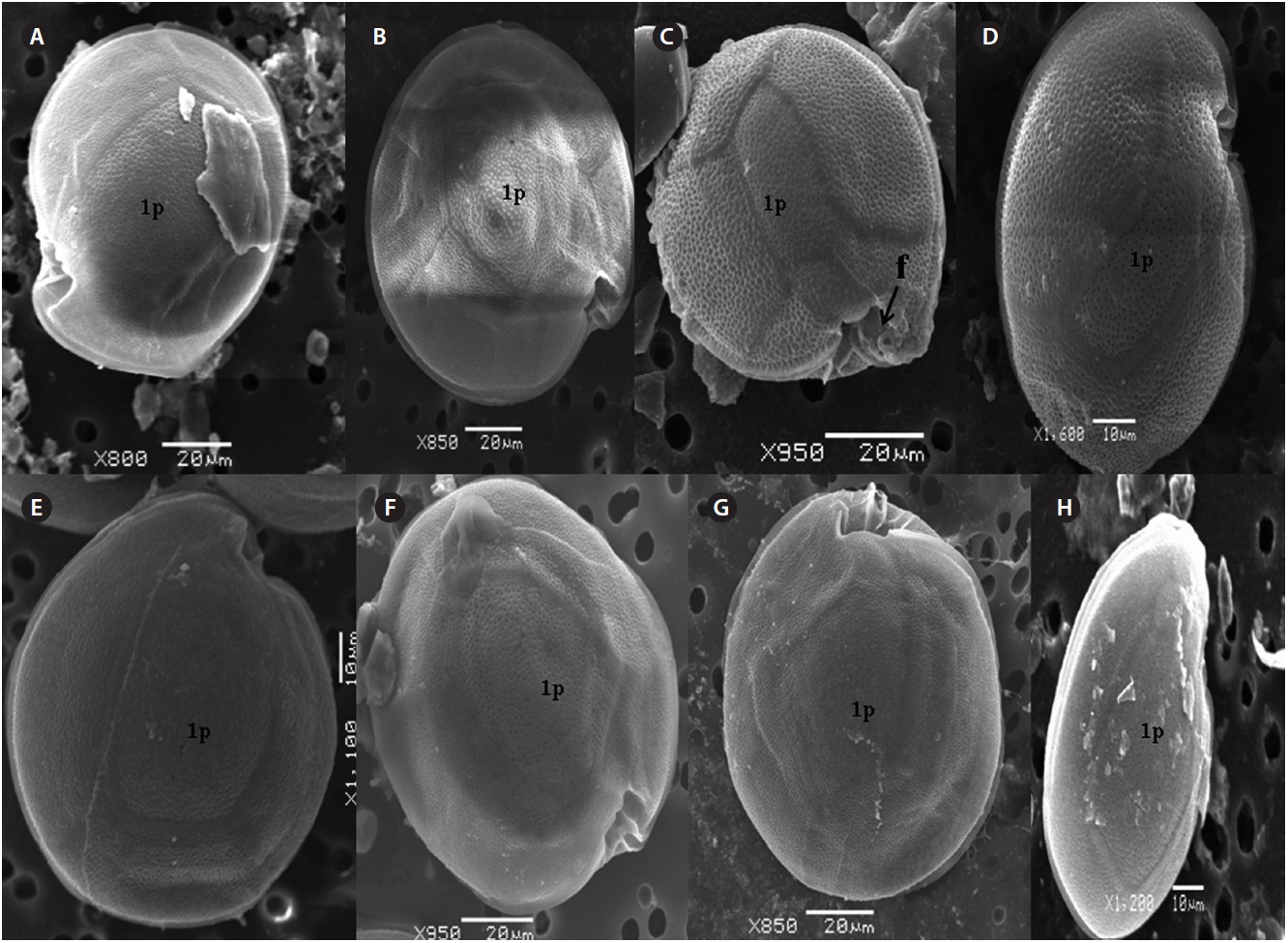
The hypotheca is more excavated with eight plates (5˝?, 1p, and 2˝˝). The postcingular plate 4˝? is large 32.5-54.0 μm and 13.8-23.5 μm wide. The 1˝˝ and 2˝˝ plates are small and connect with 1˝?, 1p and 5˝? plates (Fig. 2H). The larg-est plate is a posterior intercalary plate (1p) that is 45.3-59.6 μm long and 13.8-23.5 μm wide (Fig. 2H, 4A & B).
>
Gambierdiscus belizeanus Faust 1995 (
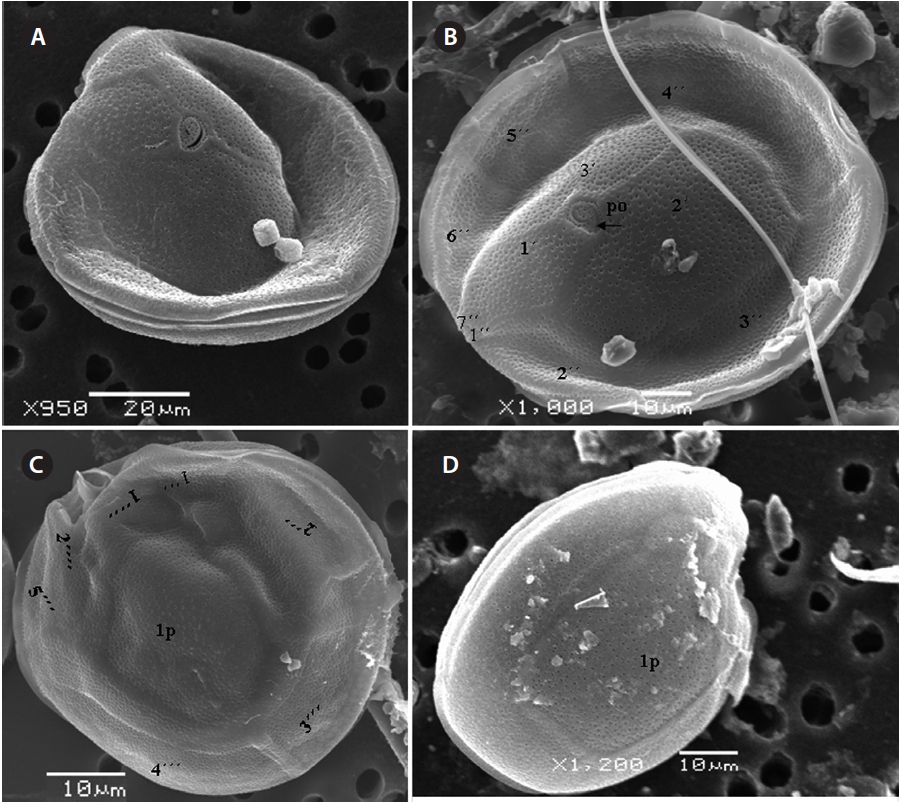
Cells of
The hypotheca is more excavated consisting of eight plates (5˝?, 1p, and 2˝˝) (Fig. 5D). The postcingular 4˝? is elongated and 40.1-49.9 μm long and 18.8-24.7 μm wide. The 5˝? is the smallest plate, and 1˝˝ and 2˝˝ are located along the edge of the 5˝?, 1˝? and 1p plates (Fig. 5D). The curved end of the cingular list at the sulcus forms a cham-ber like opening (Fig. 5D & E). The largest posterior 1p is
>
Gambierdiscus australes Faust et Chinain (
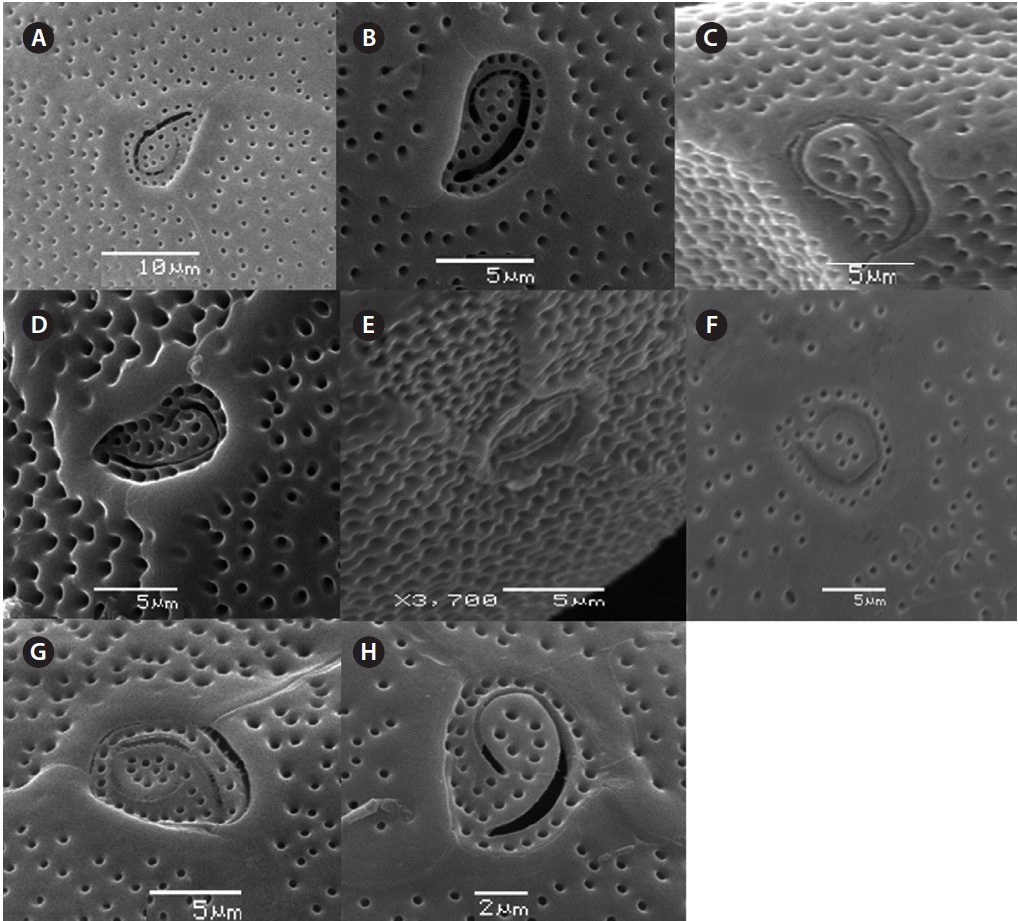
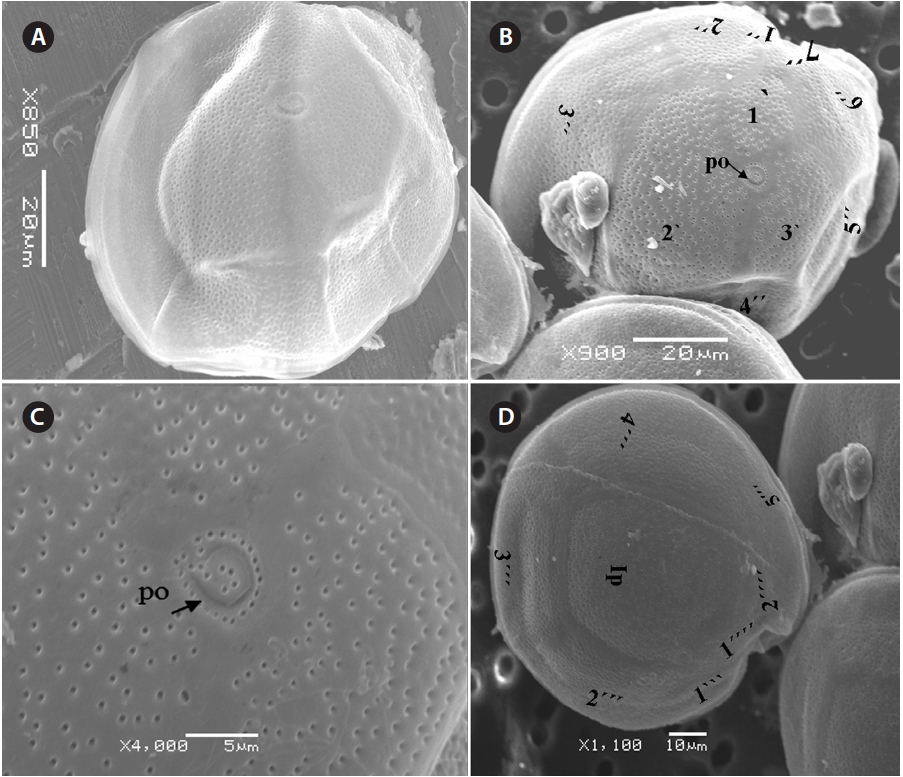
The cell is round, ellipsoid and compressed dorso-ventrally in apical view. The epitheca and hypotheca are smooth. Cell size is 60.0-81.6 μm in dorsoventral diam-eter and 48.3-75.8 μm in transdiameter. The epithecal plates are Po, 3?, 7˝ and hypothecal plates are (5˝?, 1p, and 2˝˝) (Fig. 6B & D). The Po is broadly ellipsoid, fish hooked shaped and small 5.4-6.4 μm long and 4.31-5.2 μm wide surrounded by 31 pores oriented ventrally (Fig. 3F & 6C). The apical plate 2? is longer 41.3-46.6 μm and wider 23.4-27.8 μm than those of the 1? and 3? plates. The precingular plates (1˝-7˝) has 4˝ and 3˝ plates that are larger than 1˝, 2˝, 5˝, 6˝, and 7˝ plates. The cingulum is deep, narrow and consists of Fig. 6C cingular plates and a cingular list (Fig. 6B).
The sulcus consists of eight sulcul plates. The hypothe-cal plates 1˝˝ and 2˝˝ are smaller in series and 4˝? is a large plates 31.5-35.7 μm long and 12.6-13.4 μm wide in the postcingular plate series. The 1p plate is long and narrow 25.3-45.2 μm long and 17.7-31.0 μm wide (Fig. 4E, F & 6D) and contacts the 2˝?, 3˝?, 4˝? plates. The 5˝? and 1˝? are the smallest plates and positioned close to the cingulum (Fig. 6D).
>
Gambierdiscus polynesiensis Chinain et Faust (
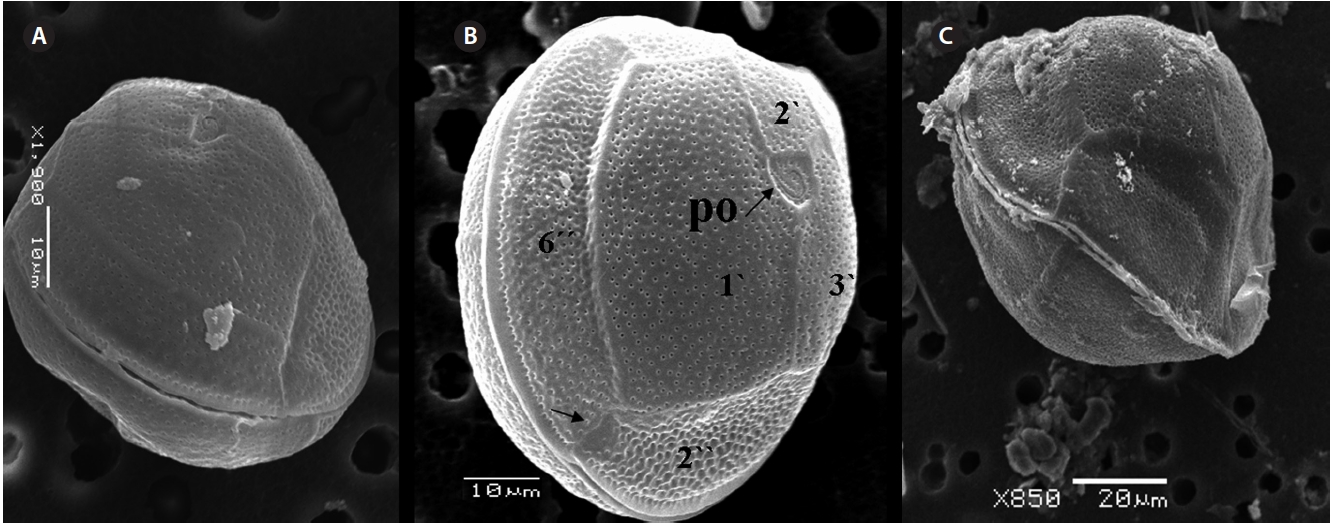
The cell is round, ellipsoid and compressed dorsoven-trally in apical view. Both thecae are smooth and convex. Cell size is 65.1-88.6 μm dorsoventral and 56.5-75.0 μm in transdiameter. Epithecal plates Po, 3?, 7˝ have a differently
triangular shaped Po that is 7.1-8.6 μm long and 5.7-6.2 μm wide, oriented ventrally, and surrounded by 38 pores (Fig. 3G, H & 7B). The apical pore 2? is a plate 45.2-48.2 μm long and 25.8-26.8 μm wide. The precingular plates, seven in number, run counterclockwise just below the 1? and consist of the 4˝ and 3˝, which are the largest plates and 2˝, 5˝, and 6˝ which are the same size, and 1˝ and 7˝, which are the smallest (Fig. 7B).
The hypothecal plates are 5˝?, 1p and 2˝˝ (Fig. 7C). The 1˝˝ plates are smaller in a series positioned parallel to the 1p plate at a dorsoventral axis wheareas 2˝˝ is larger than that of 1˝˝. The 4˝? is a large plate, 60.0 μm long and 18.4 μm wide, and the 1p plate is broad, pentagonal, 46.2 μm long and 35.5 μm wide (Fig. 4G, H, 7C & D) and contacts the 2˝?, 3˝? and 4˝?. The 5˝? and 1˝? are smallest and posi-tioned close to the cingulum.
>
Gambierdiscus cf. yasumotoi Holmes 1998 (
Cell is globular shaped in apical view. The thecal sur-face is smooth with numerous pores. Cell size is 49.25-51.93 μm long and 43.21-51.4 μm wide. The Po is fish hooked shaped is 4.7-7.7 μm long and 4.2-5.2 μm wide and positioned centrally, and connected with the 3?, 2? and 1? plates (Fig. 8A-C). The precingular plates are seven in number and 6˝ is the largerest precingular plate and 1˝ and 7˝ are the smallest. A small depression is present, enclosed by a small list from the sulcus to the right suture of the 1˝ plate (Fig. 8B). The cingulum is lipped, covered with a row of marginal pores (Fig. 8B & C).
This is the first report on the potentially toxin produc-ing species of the genus
Toxic dinoflagellate species are a diverse population in the Indian Ocean along the adjacent areas of Indian and Pakistan. Many of potentially toxic species have been col-lected in the areas where 50-90% corals had died or the turf covered coral reefs area. This is common throughout much of the region in Indian Ocean such as shallow reefs in Seychelles, Comoros, Madagascar and Chagos and reefs areas in the Gulf of Mannar, Gulf of katuch, Anda-man and Lakshadweep Island (Quod 1999, Rajasuriya et al. 1999). Only small coral patches were found in turbid areas of Pakistan (Rajasuriya et al. 1999) but very little information has been available on the coral habitat in Pakistan coastal area especially the Astola and Churna Island along the Balochistan coast (Siddiqui et al. 2008). No research is available regarding corals from Sindh coast of Karachi, which is highly influenced by pollutants, sew-age and domestic water from freshwater inlet of the Layri River.
During phytoplankton net sampling on the Karachi coast, only