Tons of toxic chemicals are released into water bodies, and their high concentrations have an enormous impact on living organisms in the aquatic ecosystem. Heavy metals are considered a serious threat to aquatic organisms in particular, and these metals have the ability to accumulate in the biota and natural environment (Levy et al. 2008). Cadmium (Cd), lead (Pb), and nickel (Ni) are among the heavy metals that are toxic to organisms in the aquatic ecosystem. In addition, a new class of toxic chemicals, endocrine disrupting chemicals (EDCs), that are commonly used in the manufacture of pesticides, plastics, and fire retardants, have resulted in changes in the nature of the pollutant burden on the aquatic ecosystem. Hence, there is considerable concern over the environmental occurrence of EDCs, because they have the potential to modulate or disrupt the synthesis, secretion, transport, binding action, or elimination of hormones in the body, thereby affecting homeostasis, development, reproduction, and the behavior of aquatic organisms (Tarrant 2005).
Dinoflagellate algae are a large group of freshwater and marine protists. About half of all dinoflagellates are photosynthetic and, therefore, they play a crucial role in the aquatic ecosystem (Taylor 1987). Alterations in the algal population due to external environmental factors, such as variations in water temperature and toxic chemical discharges, can have serious implications for water quality and on the community structures of higher trophic organisms, because algae are an important source of energy (Imhoff et al. 2004). Hence, algae based bioassays are commonly employed in environmental risk assessment for evaluating the toxicity of heavy metals and novel class environmental contaminants, and in forming regulatory guidelines (Stauber and Davies 2000). Toxicity tests are carried out by measuring growth rate or cell densities of tested algal species (OECD 2006).
Green algae and diatoms are widely-used for toxicity assessments (Moreno-Garrido et al. 2000). These include
In the present study, we used the marine dinoflagellate
As test toxicants, we used two heavy metals (Cu and Pb) and two EDCs: bisphenol A (BPA) and Aroclor 1016 (a polychlorinated biphenyl, PCB). Test concentrations of each toxicant were chosen by considering the median effective concentration (EC50) values reported from other aquatic organisms, such as algae, copepods, and fishes (Millan de Kuhn et al. 2006, Monteiro et al. 2011). A range of nominal chemical concentrations was prepared for Cu (CuSO4, Cat. No. C1297; Sigma-Aldrich, St. Louis, MO; 0.05, 0.2, 0.5, 1, 2.5, 5, 10, 25, 50, 100, 200, and 500 mg L-1) and Pb (PbCl2, Cat. No. 268690; Sigma-Aldrich; 0.05, 0.2, 0.5, 1, 2.5, 5, 10, 25, 50, 100, 200, 500, and 750 mg L-1).
For BPA (Cat. No. A133027; Sigma-Aldrich), the concentrations used were 0.1, 0.5, 1, 2.5, 5, 10, 25, 50, 100, 250, and 500 mg L-1, by using a stock solution. This was prepared by dissolving the chemical in 10% dimethyl sulfoxide (Cat. No. D4540; Sigma-Aldrich), and subsequent working solutions were prepared from this stock. The concentrations of PCB (Aroclor 1016, Cat. No. 48701; Sigma-Aldrich) were 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 25, 50, and 100 mg L-1; all dilutions were made from standard stock solutions.
Fifty milliliter aliquots of cell culture recovered at exponential phase were transferred into sterile tubes. The toxicants at each respective nominal concentration were dosed into the tubes in duplicate. The initial cell concentration was 2.25 × 104 ± 0.1 cells mL-1 as per Organization for Economic Co-operation and Development (OECD) guidelines (OECD 2006), and the samples were drawn for cell count and chlorophyll
>
Cell counting and chl a estimation
Cell counts and estimation of chl
The EC50-72 h and the percentile inhibition were calculated as recommended in OECD guidelines (OECD 2006). The concentration of the chemical that evoked the 50% reduction of the
EC50-72 h values were estimated by using a sigmoidal dose-response curve, and were plotted using Origin ver. 8.5 (MicroCal Software, Northampton, MA, USA) based on the following equation: Sigmoidal (Log EC50) = a + (b - a) · (1 + 10(x - c))-1 (Mensink et al. 2008). In addition, EC5, EC10 and EC20 values were calculated from a dose-response curve derived using Origin ver. 8.5.
>
Bioavailability of the chemicals
Bioavailability of the test chemicals was calculated based on the concentration maximum (C
Cmax = Cpk (e(-Ke · T))-1
where C
Cmin = Cave (T · e(-Ke · T))-1
where C
Decrease in cell counts and chl
>
Experimental setup and measured endpoints
The evaluation of toxicity of metals and metal-conjugated compounds to algae is important from an ecological point of view. Although trace metals such as Cu are essential for the growth of these organisms, at high concentration they prove to be fatal (Monteiro et al. 2011). Moreover, in natural environments, the physicochemical form of the metal determines the bioavailability of the metal (Franklin et al. 2000). Bioavailability of heavy metals is controlled by several factors, including pH, redox potentials, salinity, and the presence of chelators (Campbell et al. 2002). Therefore, to establish an organism as a suitable bioindicator, it is important to standardize the culture conditions and experimental endpoints. In the present study, we cultured the test dinoflagellate
Two endpoints (cell counts and chl
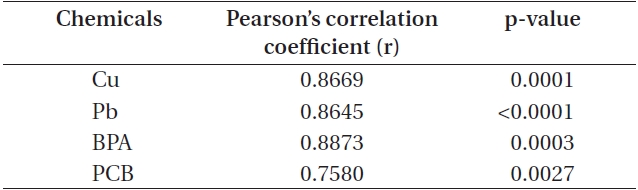
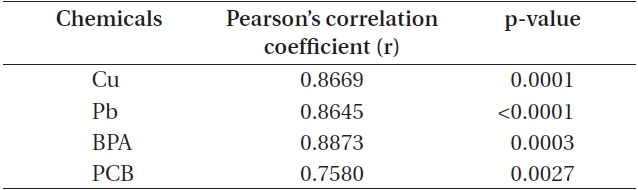
Correlation between cell count and chlorophyll a levels in Cochlodinium polykrikoides cells exposed to chemical toxicants
>
Metal toxicity on C. polykrikoides
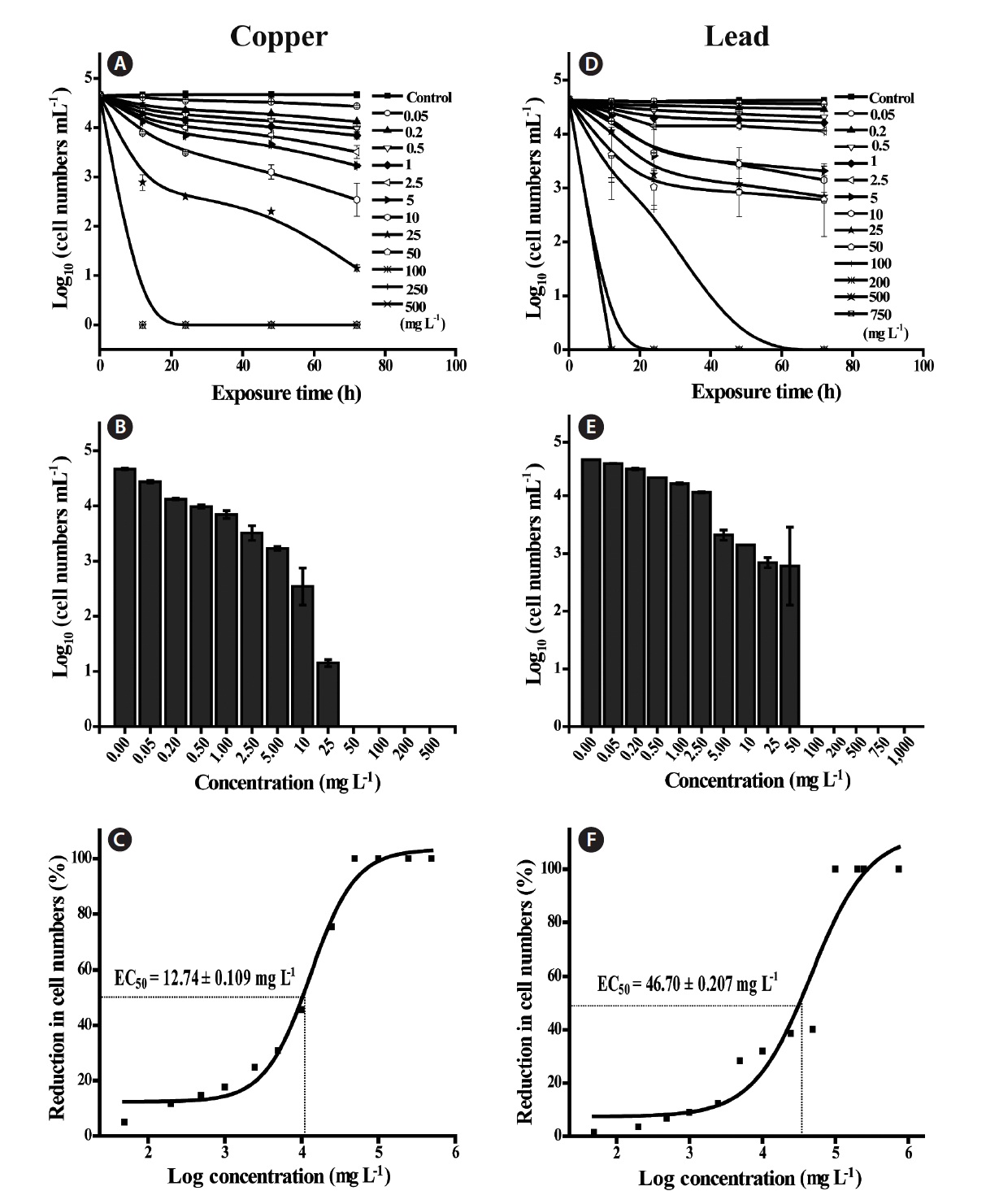
The concentrations of Cu tested ranged from 0.05-500 mg L-1, and the initial concentrations (0.05-10 mg L-1) did not show a significant change in biomass, but concentrations over 25 mg L-1 showed a significant reduction (p <
0.0001) in cell counts and chl
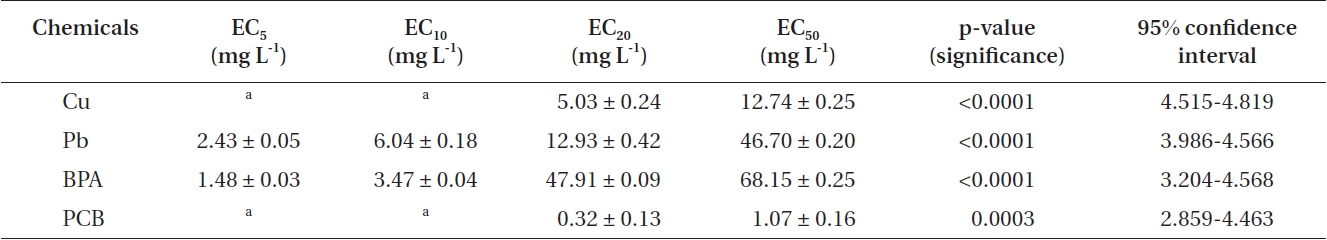
In addition, EC50 values were calculated by using sigmoidal dose-response curves that were estimated from cell counts (Fig. 1C & F). As for threshold effect parameter, we calculated additional EC5, EC10 and EC20 values, which represented the initial concentration of the test chemical that affected the dinoflagellates (Table 2). EC50 values of Cu and Pb in
>
EDC toxicity on C. polykrikoides
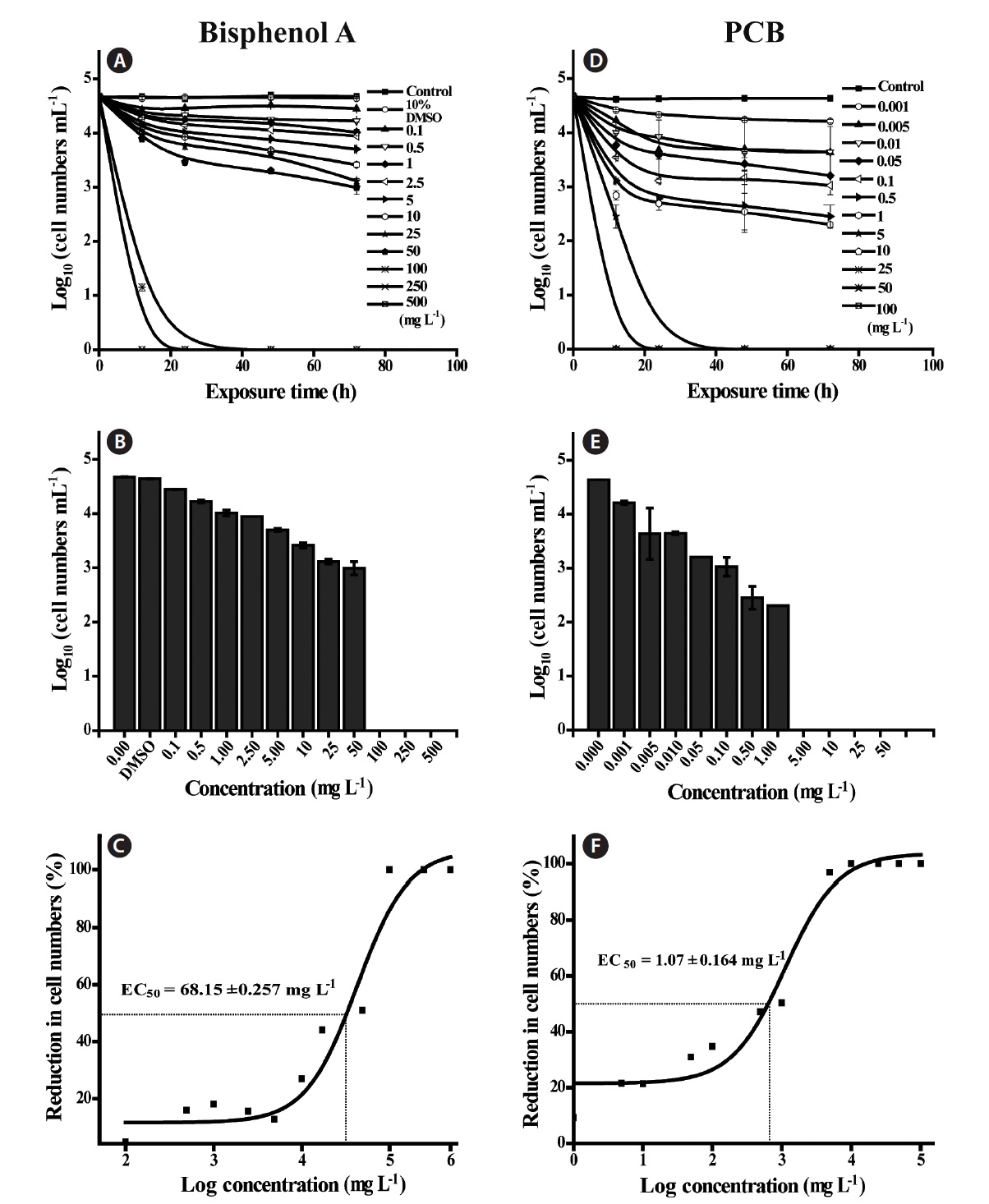
Additionally, we assessed EDC toxicity on the dinoflagellate
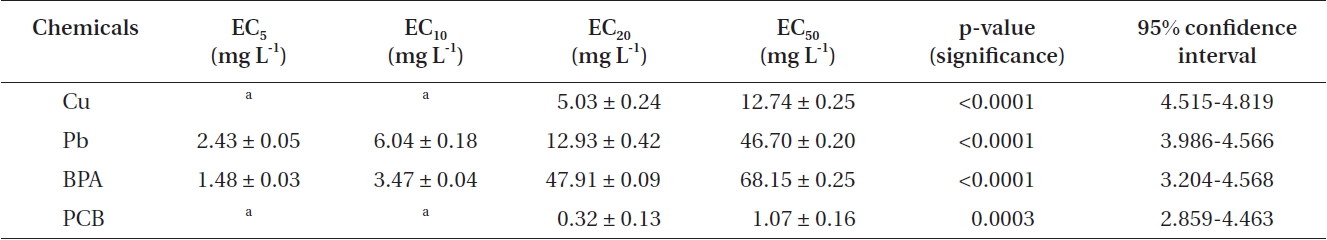
EC5, EC10, EC20, and EC50 values of heavy metals and EDCs exposed to Cochlodinium polykrikoides
of PCB concentrations exceeding 5.00 mg L-1 (Fig. 2C & D). As noted previously, since algae do not possess endocrine organs or specific systems, they may be little affected by exposure to EDCs, compared with the adverse abnormal effects of EDCs on higher organisms (Vazquez-Duhalt et al. 2006). Recently, Perron and Juneau (2011) reported that the photosystem II energy flow in green algae such as
In addition, we calculated the EC50 of PCB and BPA based on the cell counts (Fig. 1C & F), of which values were 68.15 ± 0.257 mg L-1 for BPA and 1.07 ± 0.164 mg L-1 for PCB, respectively. According to previous studies (Li et al. 2009, Liu et al. 2010), the EC50 values of BPA for the diatoms
>
Bioavailability of tested chemicals
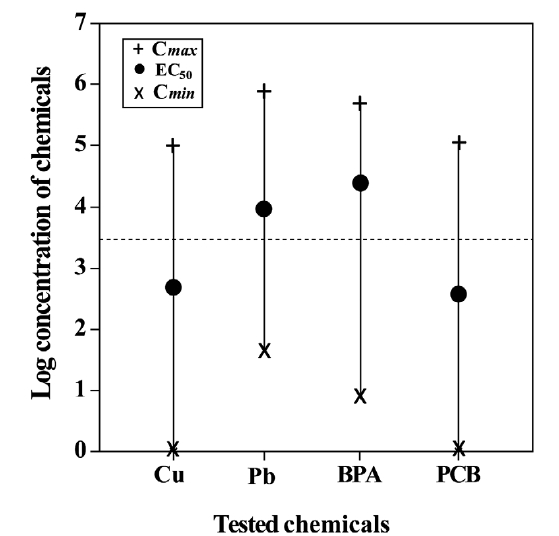
In toxicity tests, the total dose administrated need not necessarily be correlated to the total dose available to the organism (Monro 1992). Especially, it should be considered in toxicity assays by using metals and metal-conjugated compounds, as described previously. Moreover, toxicity assays performed with marine organisms and in the marine environment may be more complicated, because many complex chemical reactions occur (Jenner et al. 1997). Hence, it becomes necessary to determine the bioavailability of the tested chemicals. In the present study, we calculated the maximum concentration (C
and minimum concentration (C
test species is actually exposed (Marzo et al. 2004). This could help us in determining an approximate value for both bioavailability and effective range of a particular chemical to the test organism, as pointed by Saghir et al. (2006). The present data positioned the
In summary, the marine dinoflagellate