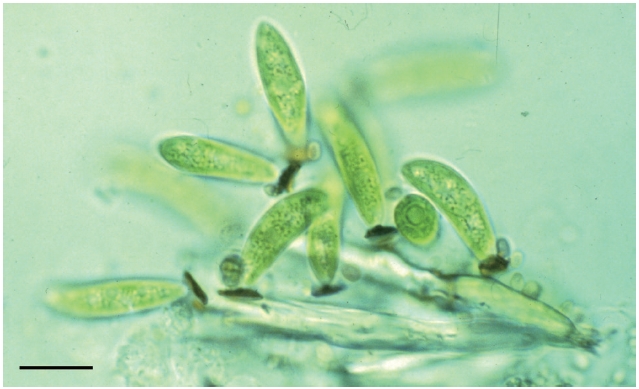
During an investigation of the chrysophyte alga
Extra-cellular biomineralization occurs in many algal groups: the Chrysophyceae (Preisig 1986) including colorless forms (Lee and Kugrens 1989), the Synurophyceae (Siver 2003), the Prymnesiophyceae (Nicholls 2003), the Euglenophyceae (Dunlap and Walne 1985, Rosowski 2003), the Chlorophyceae
Characterization of mineralized structures is an important prerequisite to experimental studies on mechanisms of biomineralization in the algae. As the mineralization in
Living unstained cells were observed with a Zeiss Axiophot photomicroscope (Zeiss, Gottingen, Germany) equipped with differential interference contrast optics. Photographs were taken on Kodak Technical Pan 2415 35 mm film (Kodak, Rochester, NY, USA). Stalks bearing mineral deposits on stalks were studied by examining cells which had been affixed to coverslips, air-dried, mounted on aluminum stubs, and observed in a scanning electron microscope (Stereoscan 250 Mk3; Cambridge Instruments, Cambridge, UK) equipped with a Link AN10/55 EDS (Oxford Instruments, Oxford, UK).
Light microscope observations of untreated wet mo- unts showed the presence of orange-red granules at the base of the
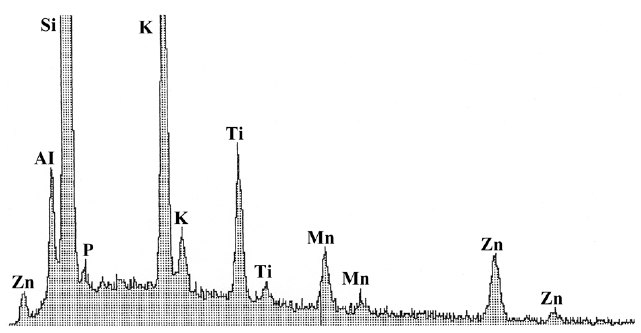
When the base of the stalks were examined with the scanning electron microscope and EDS analysis, the analyses showed manganese to be the predominant element associated with the base (Fig. 2). There was little variation in the relative proportion of Mn in the stalks from oldest to youngest. Other elements not associated with the glass or mounting stub or other parts of the cells were not detected. Iron was not detected, although low amounts could be masked by the Mn peak. Similarly other elements such as calcium were not evident.
Extracellular deposition of Mn in other algal groups has been reported in the Volvocales (Schulz-Baldes and Lewin 1975), the Charophyceae (Raven et al. 1986), and the Chrysophyceae (Dunlap et al. 1987). The lack of other minerals in the granules might be due to on the amount of iron or calcium in the culture medium, as has been demonstrated in the chrysophyte
The prevalence of Mn in the stalk of
or oxide.
This study supports the Eustigmatophyceae bear further investigation for the presence of metal elements in the remaining eight genera that produce mucilage but lack a stalk. As Santos (1996) points out, the chemical composition of the cell walls of the Eustigmatophyceae, and I can now add the mucilage, remain unclear. The presence of Mn on the stalks of