Feeding activity of waterbirds is of ecological inter-est, because the ability of parents to secure food for their broods is linked to reproductive success (Hafner et al.1993), and foraging success affects survival of both adult and juvenile birds (Frederick and Spalding 1994). Thus, foraging is directly related to ecological fitness for sur-vival, and, hence, to factors that control bird population trends. Understanding factors affecting foraging activity can provide important information for evaluating the life history of birds.
Several ecological studies have been conducted on foraging of herons and egrets. Feeding behavior and ef-ficiency of ardeids are affected by several factors, includ-ing prey density and availability (Draulans 1987, Richard-son et al. 2001), time of year (Erwin 1985) and day (Fasola 1984, Kersten et al. 1991), bird age (Quinney and Smith 1980, Cezilly and Boy 1988, Papakostas et al. 2005), weath-er conditions (Quinney and Smith 1980), habitat charac-teristics (Maccarone and Parsons 1994, Dimalexis et al. 1997), hydrological regimes including tidal cycles (Sawara et al. 1990, Strong et al. 1997, Matsunaga 2000), and social behaviors (Wiggins 1991, Master 1992). Additionally, the spatiotemporal variation in feeding efficiency of ardeids may reflect a difference in habitat quality and/or their physiological needs for nesting and survival (Dimalexis and Pyrovetsi 1997).
The grey heron (
rea and also occurs throughout the year, although their numbers decline during the winter (Lee et al. 2000). Grey herons are typically found in and around shallow water, along watercourses and shorelines, and usually in loca-tions with roost trees nearby. Availability and richness of food are important factors influencing the distribu-tion and habitat selection of foraging birds. As herons often forage far from nesting or roosting sites, they use various habitats (or patches) within their foraging range. Although their habitat preference varies among regions and seasons, they prefer shallow areas of rivers and res-ervoirs as feeding habitats in inland areas of Korea (Lee et al. 2000). In particular, some agricultural reservoirs provide good feeding habitat for grey herons (Choi et al. 2007). Although grey herons may play an important role as a bioindicator to monitor changes in local and regional environments in Korea, there is still a lack of information regarding their feeding behavior and ecology.
In this study, we compared the feeding efficiency be-tween adults and recently fledged juvenile grey herons during the late breeding season. Second, we analyzed the effect of time and space on grey heron feeding activity in a reservoir, because the distribution and density of fish may differ with time and microhabitat. Finally, as the demand for food may vary according to breeding stage, we also analyzed the effect of breeding stage on feeding efficiency in grey herons.
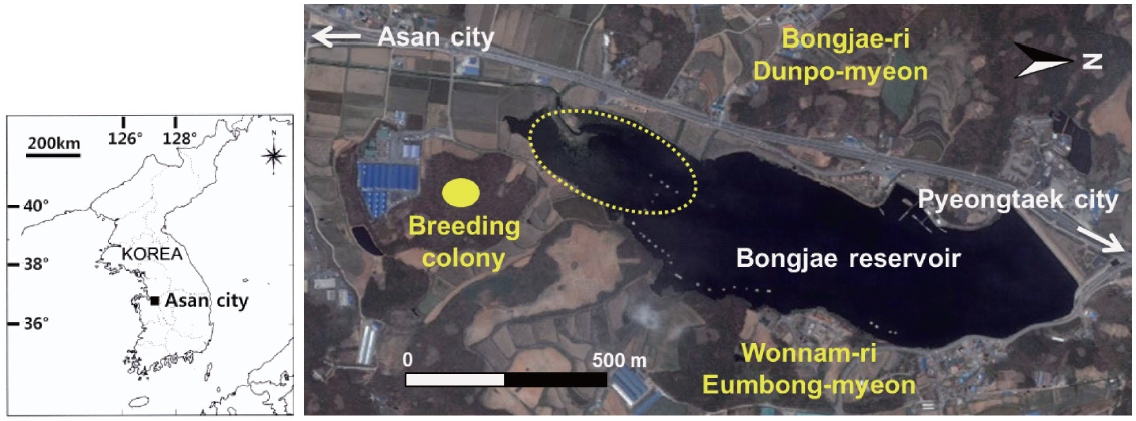
This study was conducted at a reservoir (36°52?34.6? N, 127°01?53.9? E) in Asan city, Chungcheongnam-do, South Korea (Fig.1 ). The reservoir was constructed for agricul-tural use and has been mainly used for sport fishing. This reservoir is small (38.6 ha) and comprises several mi-crohabitat types, including open water, submerged and emergent vegetation, and artificial construction such as fishing plates and buildings. Most grey herons foraging in this reservoir nest in the deciduous forest near the reser-voir. During our study, 90-110 pairs of grey herons nested in the area, in a mixed-species colony, together with great egrets (
The feeding behavior of grey herons was studied from April to early July in 2006 and 2007. Data were collected mainly from adult herons throughout the study, but data collecting of juveniles started in early June, when they first appeared at the study site. Daily observations were made from 07:00 to 19:00 h, under favorable weather conditions (no rain or string wind). Feeding activity of each heron was recorded using digital video cameras (Sony DCR TRV-20 and DCR HC-40; Sony Electronics Inc., Tokyo, Japan) or was observed directly with a 20-60× spotting scope. One observation bout lasted more than 8 minutes based on the bird’s activity, and, finally, 190 observations totaling 2,090 minutes (average, 11.0 min/individual) for adults and 31 observations totaling 313 minutes (average, 10.1 min/individual) for juveniles were recorded.
The following information was recorded for each ob-servation: age class, date, time of day, microhabitat type, observation duration, number of steps during feeding activity, feeding attempts (pecks), success (captures), and prey size captured by each heron. Moving rate (steps/min) was estimated by dividing the total number of steps by the observation duration. The number of pecks and captures was divided by the duration of observations to calculate pecking rate (pecks/min) and capture rate (cap-tures/min), respectively. Successful feeding rate was cal-culated as captures/peck. In this study, most grey herons fed mainly on fish. We recognized several fish species in the study reservoir, and the dominant species were
Four variables representing potential effects on for-aging activity of grey herons were examined: age class, calendar date, time of day, and microhabitat type. Age class was distinguished in adults and juveniles accord-ing to their plumage type. Time of day was divided into three levels: early (07:00-11:00 h), middle (11:00-16:00 h), and late (16:00-19:00 h). Calendar period had three lev-els, which were based on the nesting stage of the breeding population: 1 April-10 May (incubating stage), 11 May-10 June (nestilng stage), and 11 June-10 July (late nestling and fledging stage). Four microhabitat types were dis-tinguished: 1) open water, where herons often stood and walked; 2) vegetated, where birds foraged within densely vegetated emergent plants such as reeds (
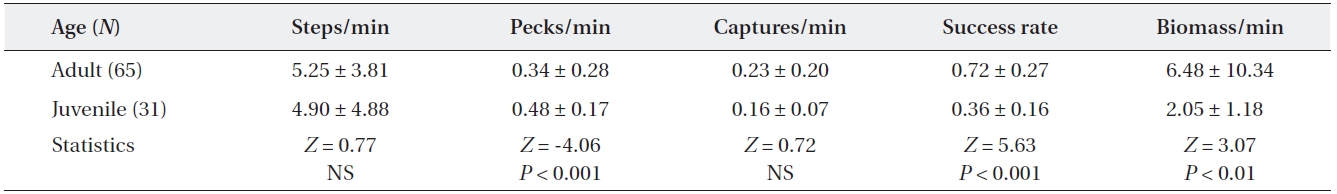
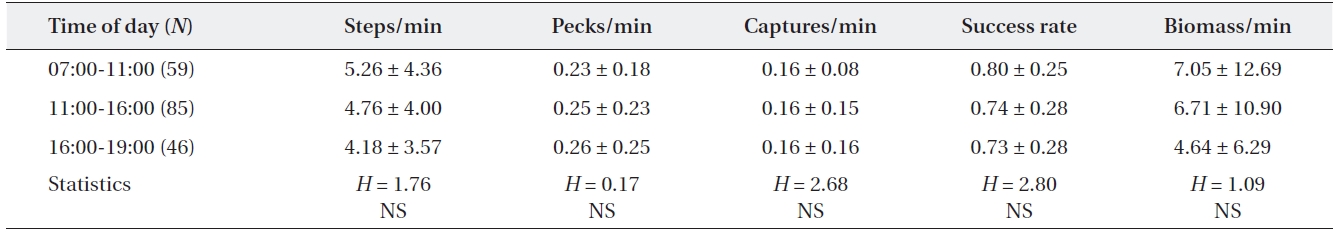
[Table 1.] Comparisons of feeding efficiency between adult and juvenile grey herons
Comparisons of feeding efficiency between adult and juvenile grey herons
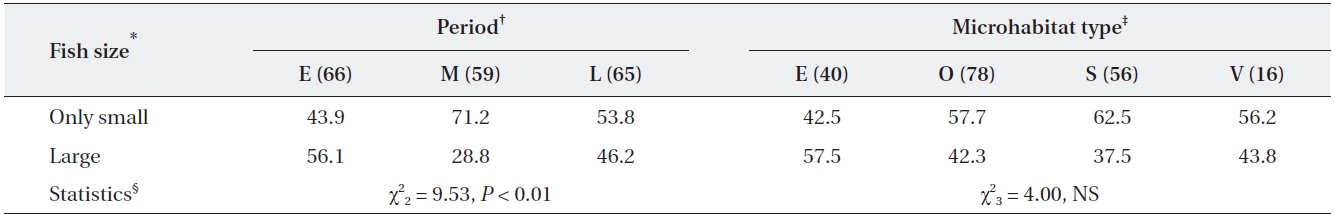
All statistical analyses were performed using STATIS-TICA (StatSoft Inc. 2004) following the guidelines of Zar (1999). None of the feeding activity variables followed a normal distribution (Shapiro-Wilk test); therefore, com-parisons of feeding activities were analyzed with the non-parametric Mann-Whitney U and Kruskal-Wallis tests, depending on the number of factor levels. Multiple pairwise comparisons were conducted with Dunn’s test after a statistically significant Kruskal-Wallis test. To analyze prey size preference, two categories (small, < 1/2 of bill size and large, > 1/2 of bill size, respectively) were divided and trends were analyzed with contingency tables (chi-square test). In most cases, the large fish were
The comparison of feeding activity between adult and juvenile grey herons is shown in Table 1. Moving rate did not differ between adults and juveniles (Mann-Whitney U test,
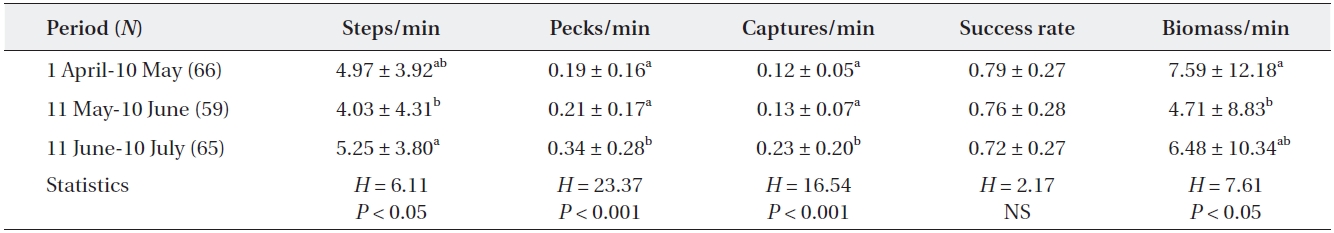
A significant difference was observed in the heron’s moving rate according to breeding stage. Adult grey her-ons walked more during the fledging stage than during the incubating and nestling stages (Kruskal-Wallis test,
[Table 2.] Feeding efficiency of adult grey herons during three different periods
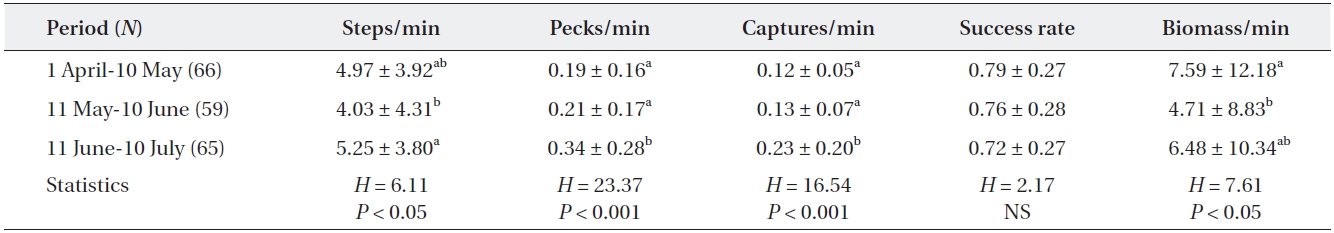
Feeding efficiency of adult grey herons during three different periods
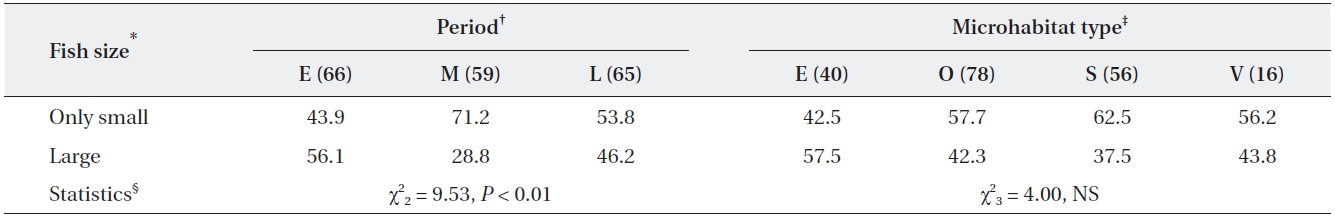
Percentage of adult grey herons that caught only small fish and large fish in relation to period and habitat type
[Table 4.] Feeding efficiency of adult grey herons by time of day
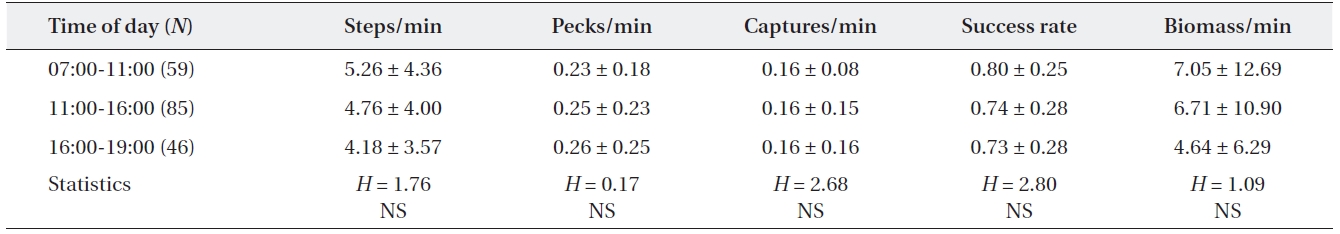
Feeding efficiency of adult grey herons by time of day
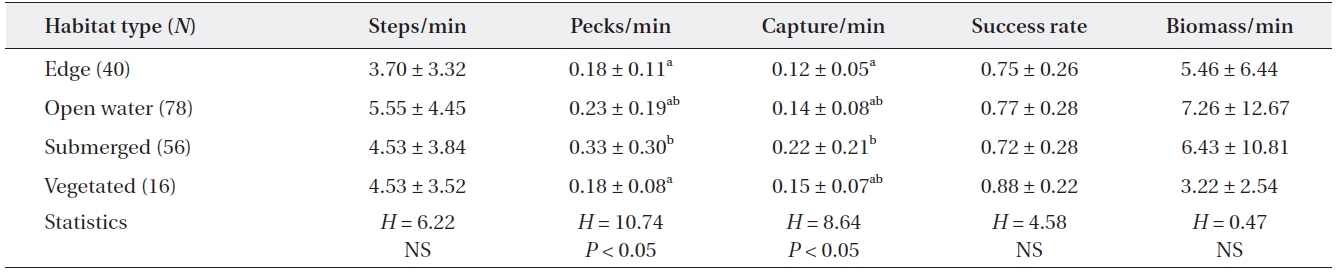
Feeding activities of adult grey herons varied signifi-cantly among the four microhabitats (Table 5). The mov-ing rate of grey herons did not differ among the four habi-tat types (Kruskal-Wallis test,
In this study, grey herons completed an average of five steps per minute when they had feed in the reservoir, and moving rate was not affected by age, time of day, or habi-tat type, although some differences were observed among periods. Grey herons usually have less conspicuous forag-ing habits than those of other ardeids, and they stand and wait for several minutes to catch prey (Choi et al. 2008). Thus, their typical feeding strategy is to catch a few large prey during the course of the day, even in the nesting pe-riod (Dimalexis et al. 1997, Kushlan and Hancock 2005).
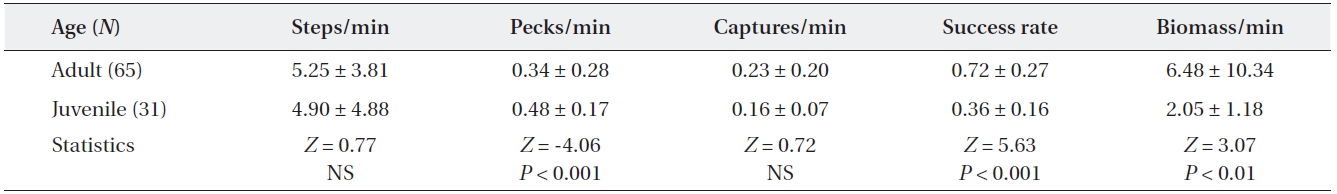
Adult grey herons were more efficient foragers than ju-veniles; adults had higher successful capture rates than those of juveniles. Juveniles attempted more pecks than adults, but many attempts failed to capture prey. Thus, juveniles attempted to capture prey more often to satisfy their daily energy demands (Brandt 1984). Less successful feeding attempts by young herons has been reported by
[Table 5.] Feeding efficiency of adult grey herons by microhabitat type
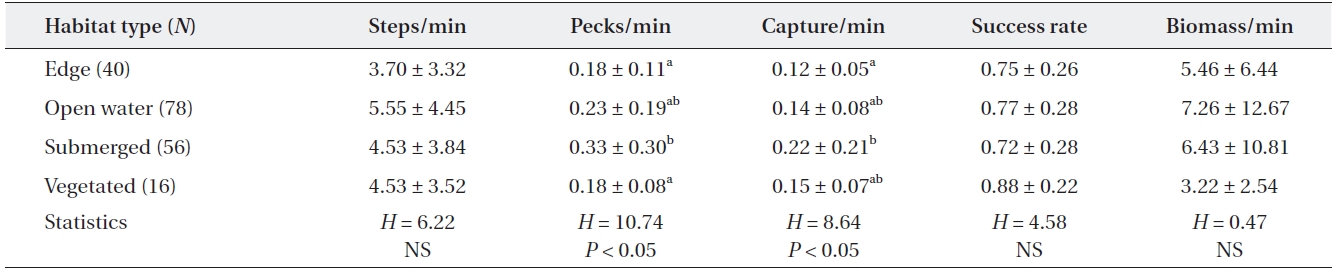
Feeding efficiency of adult grey herons by microhabitat type
several authors (Quinney and Smith 1980, Lo and Ford-ham 1986, Burger and Gochfeld 1989, Papakostas et al. 2005). The lower feeding success of young herons may result from a lack of foraging experience (Draulans and van Vessem 1985, Burger and Gochfeld 1989, Marchetti and Price 1989) and sensorimotor maturity (Cezilly and Boy 1988, Marchetti and Price 1989). Juveniles also tend to capture smaller prey than adults, because they are less skillful at catching and handling large prey and, thus, have a lower food intake rate (Quinney and Smith 1980, Drau-lans and van Vessem 1985). In contrast, adult grey herons are specialists on larger prey. Low feeding efficiencies of juveniles may affect their survival after fledging and also affect fat reserves for the autumnal migration.
Temporal changes in the feeding efficiency of herons may be related to their breeding stage (Matsunaga 2000, Papakostas et al. 2005) and may also reflect changes in prey availability within the foraging range (Matsunaga 2000, Richardson et al. 2001). In this study, most grey her-ons were incubating eggs during the early period (from April to 10 May) and, therefore, only needed food for themselves. However, as chicks hatch in May, some pairs had small chicks that required additional food; thus, the energy demand increased gradually to reach a peak in June. Our results showed that total biomass intake taken by adult grey herons was high during the incubating and fledging periods. During the incubation period, adult grey herons frequently caught large fish that were more than twice as long as their bills despite the low capture rate. Many adults caught a few large prey and then rested during this period. In April and May, big fish such as carp (
As small chicks are in nests beginning in May, adult herons must select smaller prey, because nestlings are un-able to ingest large-sized fish (Moser 1986). Adult herons frequently caught small fish in May and June. However, despite the increase in food demand for growing chicks during the nestling period, the capture rate and biomass intake of adults was lower than that during the other two periods. This was probably related to the short distance between feeding and nesting sites (within 500 m from the colony). The short distance between the feeding and nest-ing sites could shorten the food delivery time to nests and increase the food provisioning rate, even though it was small and low volume prey.
The foraging efficiency of grey herons did not vary among times of day, although feeding intensity was low at midday in this study. Many ardeids vary their foraging intensity among times of day (Erwin et al. 1985, Lo and Fordham 1986, Kersten et al. 1991). Daily variations in feeding activity mainly occur in estuarine habitats, which are affected by the tidal cycle (Sawara et al. 1990, Matsu-naga 2000) and also occur in non-tidal habitats due to the effect of daily distribution and behavior of prey spe-cies (Kersten et al. 1991). However, Sawara et al. (1990) reported continuous feeding of grey herons in non-tidal environments and Erwin (1985) reported that time of day had little influence on feeding during the breeding season in contrast to winter, because of high food demands for rearing chicks. Additionally, no daily difference in forag-ing activity may infer that the availability of prey did not differ significantly by time (Papakostas et al. 2005).
The feeding behavior and success of many ardeids is af-fected by the habitat or microhabitat type where the her-ons feed (Hafner et al. 1986, Draulans and Hannon 1988, Dimalexis et al. 1997, Maccarone and Brzorad 2002). This may be due to differences in habitat structure (Dimalexis et al. 1997) or prey availability (Richner 1986, Maccarone and Parsons 1994). Higher pecking and capture rates in submerged plant areas indicated that this area was a suit-able feeding microhabitat for herons, probably due to the high density of small fish. Submerged aquatic plants provided spawning sites for several fish and also provided protection and feeding area for small and young fish. Ad-ditionally, we found that the moving rate of adult grey her-ons tended to be higher on open water areas than in veg-etated areas, although the difference was not significant. Thick vegetated areas (emergent and submerged plants) probably prevented grey herons from walking.
In conclusion, a reservoir provided important feeding habitat for breeding herons, and the feeding efficiency of grey herons was affected mainly by the microhabitat with a small time effect. These results suggest that the distribution of fish depended on microhabitat type and that time of day had less of an effect. In addition, the dif-ference in feeding efficiency in relation to breeding stage reflected the change in food demand during the breeding period. Consequently, we found that grey herons foraging at a reservoir achieved different efficiencies in response to microhabitat and breeding stage. However, the direct effect of spatiotemporal variation in prey density on feed-ing activity of grey herons at reservoirs still remains to be analyzed.