Intensive land use for forestry and agriculture has led to a marked increase in habitat patchiness on various spatial scales in agricultural landscapes. Habitat patches may be isolated “islands” within another habitat type. For exam-ple, a forest stand can be confined to a large monocultural agricultural field, or a pasture can be located within a for-est. Habitat patchiness affects the spatial distribution of invertebrates at several spatial scales (Niemela et al. 1992, Niemela 1997, 1999, Kinnunen and Tiainen 1999).
Biological indicators can be very useful for assessing habitats if they are reasonably representative of the habi-tat since using only a few species groups, e.g., through extrapolation, is a rapid technique. A useful indicator species has been defined as one that is distributed over a broad geographic area, has specialization in certain habi-tats, provides early warning of change, is easy and cost-effective to survey, is relatively independent of sample size, has a response that reflects the responses of other species, is easily distinguishable between natural cycles or trends and those induced by anthropogenic stress, and should be of potential economic importance (e.g., Noss 1990, Pearson and Cassola 1992, Niemela 2000, Samways 2006). However, it is difficult to find species or species groups that have all of the characteristics mentioned above (Noss 1990, Pearson and Cassola 1992). Carabid beetles are widely recognized as potentially valuable indi-cators of environmental variation since they are diverse, can be easily sampled, and are sensitive to changes in the physical and biological environment (Lovei and Sunder-land 1996, Rainio and Niemela 2003).
Kang et al. (2009) reported that the composition of ca-rabid beetle fauna, dominant species, and carabid beetle assemblage patterns differed among habitats (levee, dike, hillock, streamside) in the same sites of the current study.
In this study, we aimed to propose some potential in-dicator species for representing these habitat types from the generalist and specialist aspects for different habitat types in agricultural landscapes and to investigate their suitability as indicators by analyzing their correlation with the spider group.
Carabid beetle samplings were conducted using un-baited pitfall traps in four adjacent habitat types (levee, upland dike, hillock, streamside) in three sites, Paltan-myeon, Hwaseng-si, and Gyeonggi-do, in two-week in-tervals from November 2000 to November 2002. Detailed information about the study site characteristics and sam-plings were published previously (Jung et al. 2008, Kang et al. 2009). The nomenclature and identification of carabid beetle species have also been published elsewhere (Kang et al. 2009).
Analyses were conducted of the carabid species di-versity, and a cluster analysis was conducted using the dominant species. An agrobiont species is defined as a dominant species in various habitats of the farm village area (Samu and Szinetar 2002). Twenty-two agrobiont species represented >3% of the total numbers in at least one habitat type. This list was published previously (Kang et al. 2009). The agrobiont carabid species diversity was compared among the habitat types using Shannon’s di-versity index (Pielou 1975). To compare the incidence of opportunistic species, the carabid dominance structure was investigated by construction of an agrobiont species rank-abundance plot and an agrobiont abundance distri-bution model (Niemela et al. 2002) at four habitat types using Species Diversity and Richness III (Pisces Conserva-tion 2004). Agrobionts are considered ubiquitous species that occur in a wide range of habitats and are considered typical pioneer species with a well developed dispersal capacity, especially in arable land (Samu and Szinetar 2002, Lambeets et al. 2008).
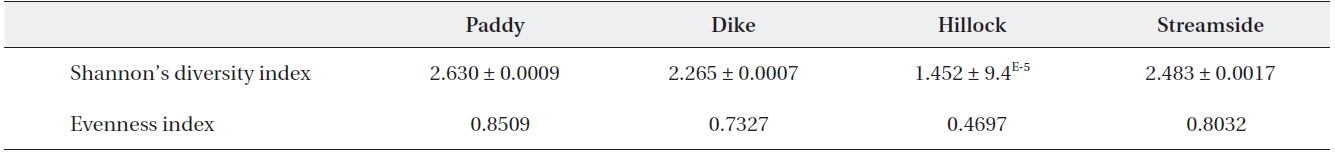
Shannon’s diversity and evenness index values among the four habitat types using 22 agrobiont carabid beetle species
We used the agrobiont species to select the indicator species and investigate the body size and wing types of the selected species as potential bioindicators to under-stand their ecological traits across landscape elements (Fournier and Loreau 2001, Niemela et al. 2002).
The relationship was analyzed between the carabid beetle and spider groups, both of which were collected in the same investigation sites to determine whether cara-bid beetles can be utilized as bioindicators. Spider group data from the Korea Science and Engineering Foundation report were used (Lee 2003).
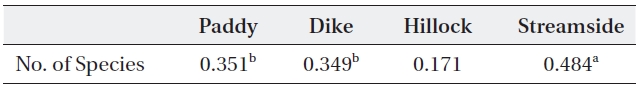
The Shannon’s diversity index values for the 22 agro-biont carabid beetle species were 1.452-2.630 among the four different habitat types (Table 1). The diversity was lower in the hillock habitat than in any other habitat type. With regard to diversity index, the value of Simpson’s Evenness was lower in the hillock habitat than in any oth-er habitat types (Table 1).
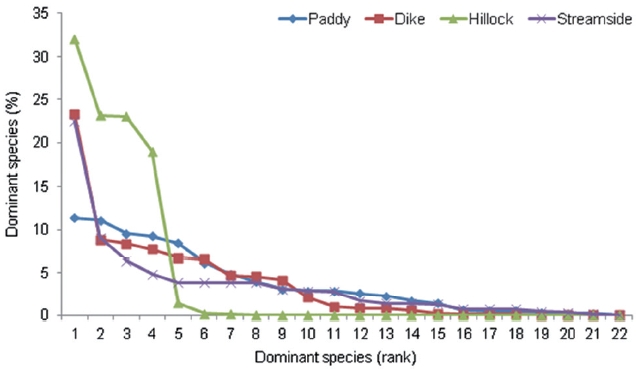
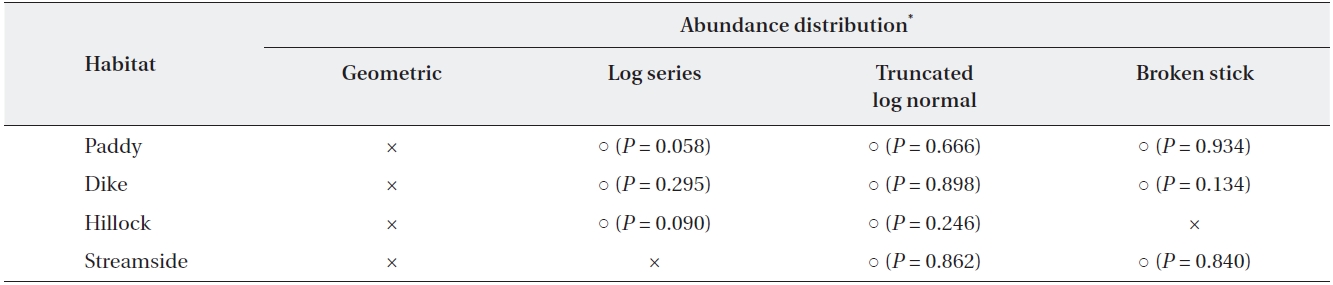
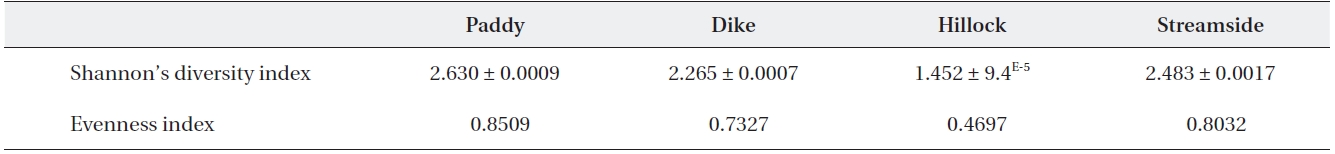
The dominance structure of the 22 agrobiont species in the carabid beetle communities of the four habitat types is illustrated using rank-abundance curves (Fig. 1). The dominance structure differed among habitat types. The structure of abundance distribution in the levee habitat highly fit a broken stick model (
[Table 2.] Analysis of abundance distribution of the four habitat types at three sites
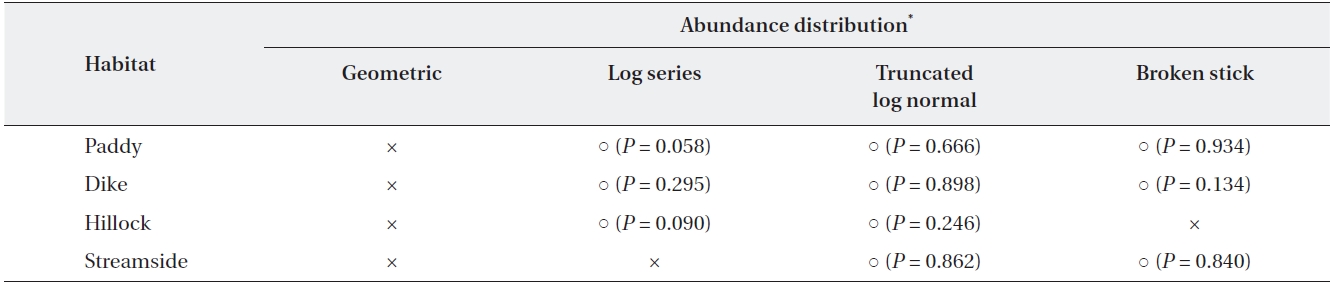
Analysis of abundance distribution of the four habitat types at three sites
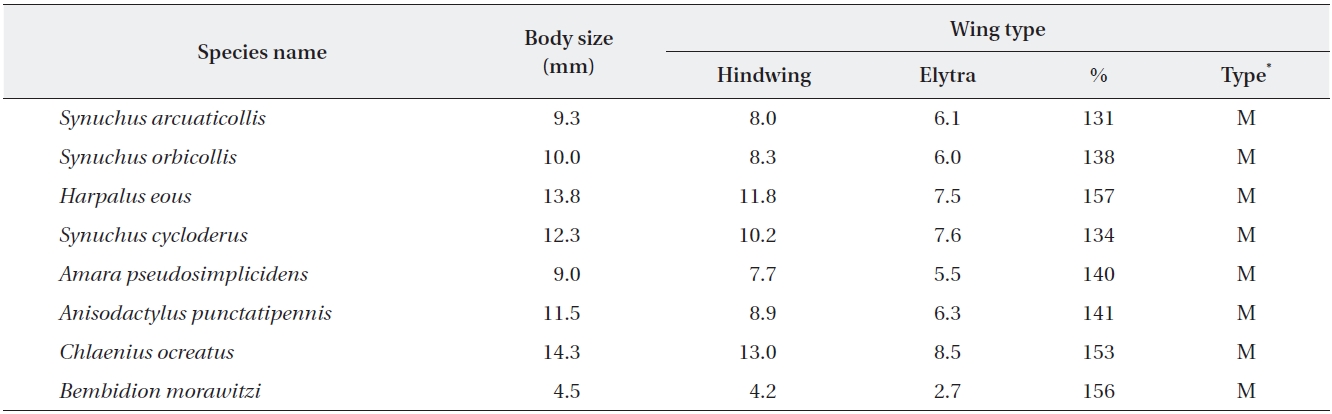
[Table 3.] Body sizes and wing types of the proposed bioindicator species in four habitat types
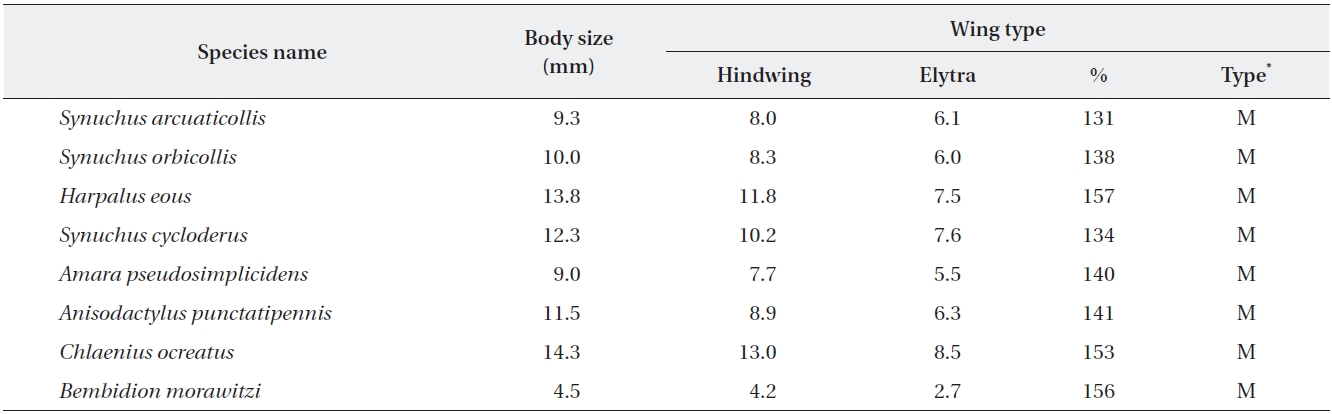
Body sizes and wing types of the proposed bioindicator species in four habitat types
cating that they are potential bioindicators.
The body size of the selected species for potential bioin-dicators was classified into three groups: small (<10 mm), medium (10-20 mm), and large (>20 mm), which can be used to examine the relationship between ecological traits and the response to disturbances for species across landscape elements (Niemela et al. 2002). The wing types of the selected species bioindicators were classified into macropterous (hindwing fully developed) according to the degree of hindwing development (Table 3), which was calculated for each species as the ratio between the wing area and the elytron area (Fournier and Loreau 2001).
Carabid communities may sensitively respond to wide environmental elements such as habitat structure, habi-tat type, and topographic distance (Weaver 1995, Dufrene and Legendre 1997). Even if a habitat environment re-mains the same, the qualitative composition of the cara-bid beetle may differ in response to the strength of neigh-boring habitat factors or according to the direction of a disturbance (Rainio and Niemela 2003). The above men-tioned species appear to have high potential as biological indicator species to abstract specific habitat types. Dif-ferences in habitat types and environment affect species composition and abundance.
The relationship between the carabid beetle group and the spider group collected at the same investigation sites was analyzed (Table 4). The correlation of species number between the carabid beetle and spider groups was modest (
Some studies have reported correlations between species richness of different species groups, e.g., tiger beetles and birds (Pearson and Cassola 1992), butterflies and flowering plants (Kremen 1992), and several insect groups and overall species diversity (Wise 1993, Duelli and Obrist 1998). However, several other studies reported no or very low correlations between species richness of different taxonomic groups (plants, mosses, birds, butter-flies, beetles, etc.) (Kremen 1992, Prendergast et al. 1993, Oliver and Beattie 1996, Lawton et al. 1998, Jonsson and Jonsell 1999). There are currently no clear answers regard-ing whether there are significant correlations between taxonomic groups, but the ecological requirements of the species and the observation scale can provide some guidance. There may be no correlation between species groups with different ecological requirements (Lawton et al. 1998, Jonsson and Jonsell 1999), whereas a correla-tion can be expected between species that depend on the same ecological factors (e.g., moisture, soil quality, and
[Table 4.] Correlation coefficient between carabid beetle and spider groups by habitat type
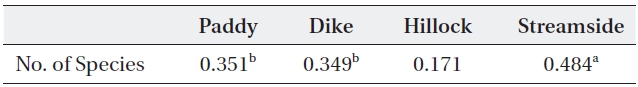
Correlation coefficient between carabid beetle and spider groups by habitat type
dead wood). Species richness also depends on the obser-vation scale (e.g., Blake et al. 1994, Weaver 1995). Further studies should be conducted to elucidate whether one type of taxonomic group can be used as a surrogate for assessing biodiversity of different habitat types.