During the breeding season, diet selection often af-fects breeding success (Pierotti and Annett 1991, Wanless et al. 2007). Parent birds have to select appropriate food, which can meet the nutrient requirements of chicks and maximize the efficiency of parental efforts. Experienced parents often select higher quality prey compared to young parents (Limmer and Becker 2009). Parents must also select prey items depending on the age of chicks, as older chicks need more food than younger ones; as such,prey selection changes in terms of composition or size as chicks age (Navarro et al. 2009, Ramos et al. 2009, Mitrus et al. 2010). Yellow-legged gulls (
Yellow bitterns (
In this study, we tested whether yellow bittern parents change diet in terms of amount and/or prey size accord-ing to chick development. We also investigated factors af-fecting the frequency of nest visits during the chick rear-ing period in yellow bitterns.
>
Study areas and collecting boluses
This study has been carried out at the artificial swamp (55 ha) in An-san city (126°50´04˝-48˝E, 37°16´ 34˝-43˝N), Republic of Korea from late May to August in 1999 and 2000. To investigate the diet of yellow bitterns during the breeding season, we collected boluses which regurgitated by chicks when they feel stressed, a behavior exhibited by yellow bitterns and other bird species of the family Aredaidae (Kirkpatrick 1940, Olmos et al. 2001). For the purposes of this study, we define a bolus as a food ball re-gurgitated by a chick in one attempt. We visited 21 nests every day during the late incubation period and during the chick rearing period to identify the age of chicks and to collect boluses. Chicks were individually marked with a non-toxic marker pen on their bills or color rings.
Once chicks regurgitated boluses, we measured the wet weight of a bolus and retrieved them from nests to identi-fy prey items and to record the number of items. Because heads of fish in a bolus are slowly digested compared to other part of the fish (Barrett et al. 2007), we counted the number of fish heads to avoid over-counting. Food items in a bolus were identified based on the guide book of fish (Kim and Kang 1993), insects (Youn 1995) and aquatic insects (Bae 1998). Prey items in a bolus were identified at species level or at family level and the number of food items in a bolus were counted. Body size of a fish in a bo-lus was measured from mouth to tail to the nearest 0.01 mm using vernier calipers. Boluses were preserved in 60% ethanol for weighing the ash-free dry weight in the labo-ratory.
For the analysis of the relationship between age of chicks and prey size, only fish of known size were includ-ed in the study. The proportion of prey items was estimat-ed as a percentage of the total number of prey in a bolus.
In the laboratory, we used ash-free dry weight (AFDW) to estimate the energy of regurgitated prey. AFDW has been used to estimate actual energy intake of prey in many previous studies of geese (Therkildsen and Mad-sen 2000) and shorebirds (Ge et al. 2009). Non-prey items such as pieces of reeds were removed to estimate AFDW of regurgitated prey. Boluses were dried at a temperature of 55°C and burnt in a 550°C oven for 5 h. AFDW was esti-mated in 62 boluses collected from 1999.
>
Frequency of visiting nests by parents
We estimated the frequency of visiting nests by par-ent birds from June to August. This estimate is in terms of nest visits per h, based on recordings made with a video camera. We recorded nests with chicks for approximately 2 h per a day between 8.30 and 17.00. A video camera was camouflaged with reeds and placed 2 to 3 m away from the nest. Items of interest in these recordings are age of the oldest chicks in a brood, date of observation, and brood size.
All data analysis was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). ANOVA was used to inves-tigate the relationship between food amount and other factors. All biologically relevant two-way interactions between explanatory variables were included in the ini-tial model and we removed the stepwise least significant term. Statistics are shown for the last step variables in-cluded in the model. The correlation between amount of food and age of chicks was examined using Pearson’s rank correlation test. This is significant when the
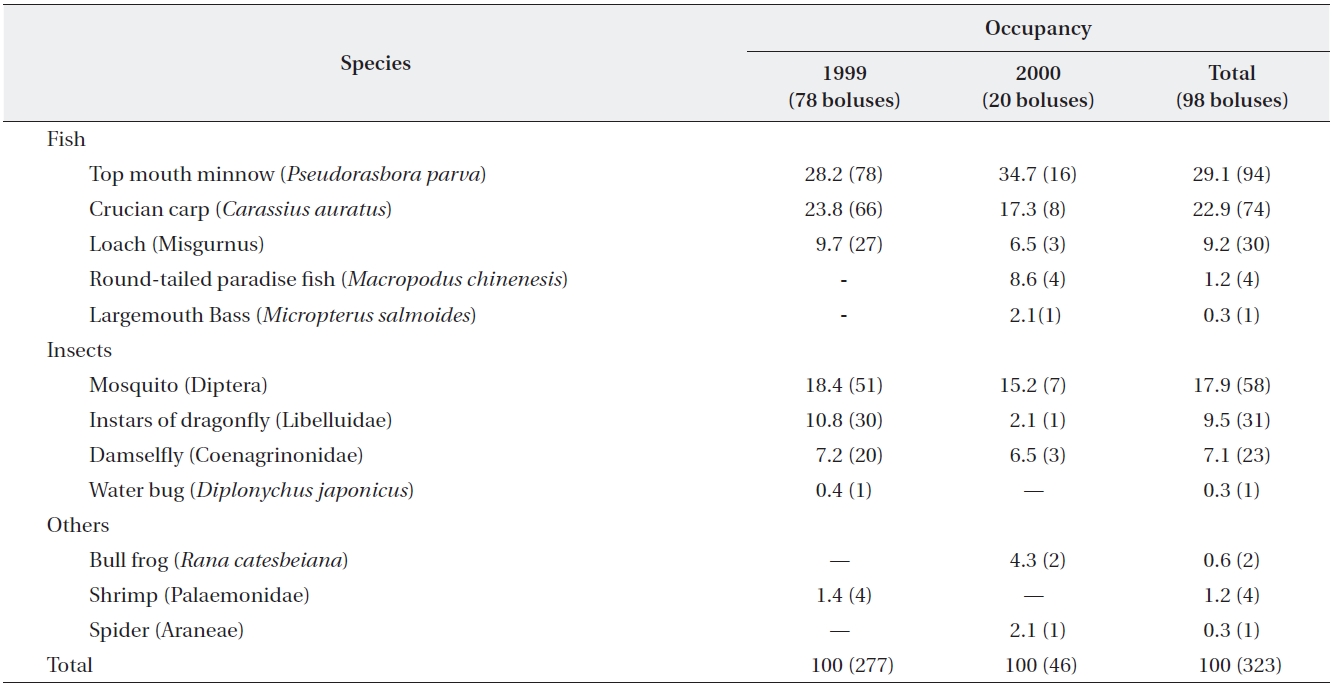
A total of 98 boluses were collected from 52 chicks aged from one day to eleven days after hatching. A mean of two boluses per a chick were taken (range, one to six boluses per a chick). Boluses were collected from June to August, 1999-2001.
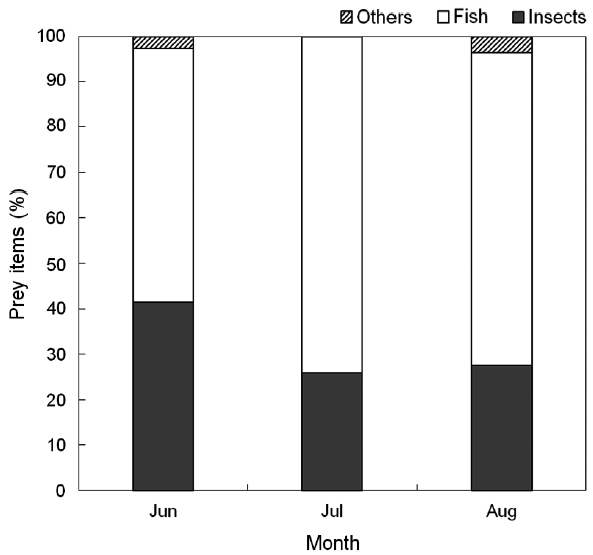
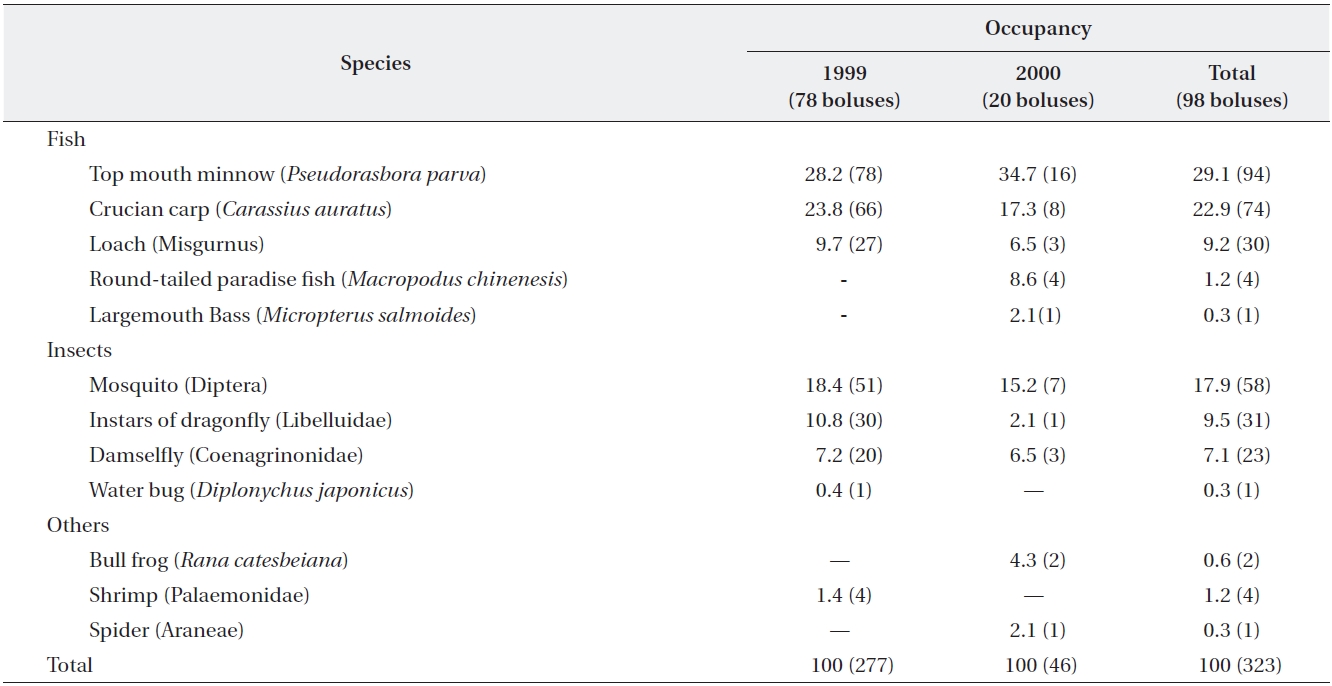
Yellow bitterns were observed to feed chicks primar-ily on fish (63%) and insects (33%) (Table 1). A bolus in-
Prey items (%) in 98 boluses regurgitated by 52 chicks of yellow bitterns in 1999 and 2000
cluded a mean of 3.8 individual items. With regard to fish in boluses, top mouth minnows (
>
Amount of food and prey size
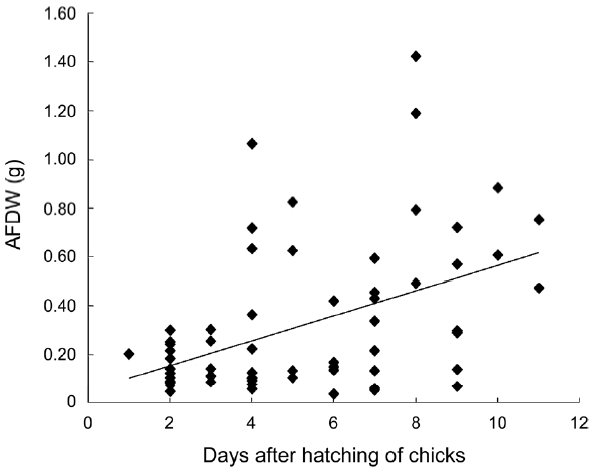
The fish size in boluses was measured in 48 fish, of which the entire body length is estimated. The mean ob-served length of fish in boluses was 35.8 ± 2.65mm (range, 15.56 to 93.79 mm). The wet weight of a bolus ranged from 0.2 g to 7.7 g and AFDW was from 0.032 g to 1.421 g. AFDW of a bolus was observed to increase with the age of chicks (

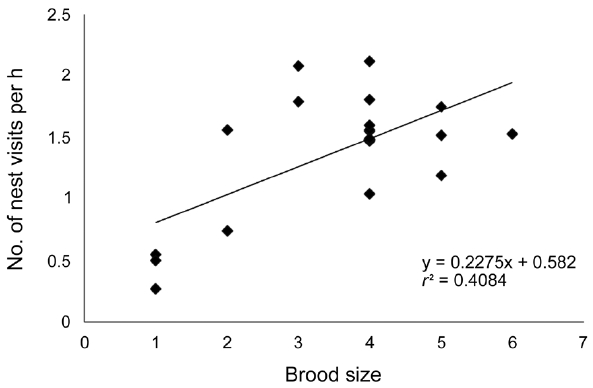
Frequency of nest visits by parents for feeding chicks in eight nests related to brood size age of the oldest chicks in a brood and date of observation
In this study, we recorded eight nests containing chicks aged three to ten days after hatching. A total of 23 obser-vations were made for a total recorded time period of 22 h 10 min 5 s. Chicks are not disturbed for more than 10 min after placing a video camera. Parents visited nests 1.39 ± 0.09 times per h (range, 0.27 to 2.12 times per h) during the chick rearing period. Parents visited more frequently when they had a larger brood in a nest (
In the results of this study, food items fed to chicks by yellow bittern parents are observed to vary throughout the breeding season. In the early breeding season, yellow bitterns feed their young on similar proportions of fish and insects, but later in the breeding season more fish are consumed than insects. This may be related to the change of food availability in the study area over time. As the fries of top mouth minnows and crucian carps, which form the main prey of yellow bitterns, hatch in April (Kim and Kang 1993), small sized fish may become more abundant later in the breeding season. In Japan, yellow bitterns are op-portunistic predators (Ueda 1992). They mainly feed fish and insects, but also reptiles and amphibians under poor food conditions (Ueda 1985, 1992).
The diet fed by yellow bitterns to chicks thus consisted of varied items such as fish, insects, and frogs. Fish form the most important prey item during the chick rearing period. In Malaysia, yellow bitterns breeding in rice fields are shown to prey more on invertebrates such beetle lar-va, blue-bottle flies, and damselflies than fish (Lansdown and Rajanathan 1993). In our results, mosquitoes were observed to be the most frequent insect prey of yellow bitterns. However, it is observed through video recording that parents usually catch mosquitoes when they are with chicks in the nest. Parent birds might catch mosquitoes to protect chicks in the nests.
The rate of food provision by parents varies with chick age and brood size (Adler and Ritchison 2011). The fre-quency of nest visits by parents in yellow bitterns is re-lated to brood size. Parent birds are observed to visit nests more frequently when they have a larger brood. This may be due to increased food requirements when bitterns have a large brood. The age of the oldest chick in a brood did not exert an observable influence on the frequency of nest visits by parents.
The wet weight of a bolus increases with a chick’s age. The parents of yellow bittern brought more food, but did not alter the frequency of nest visits as chicks grew. One possible explanation is that higher rates of nest visi-tation may increase predation risk (Eggers et al. 2005). Parents may bring larger numbers of fish in a food-load rather than visit nests more frequently in order to meet increased food demands of chicks. However, parents with larger broods may have to work harder and visit nests more frequently to maximize food quantity for chicks.
To conclude, yellow bitterns are observed to forage op-portunistically depending on food availability in habitats. The age of chicks and brood size is shown to affect the amount of food and the number of nest visits, respec-tively.